Despite the efforts made to improve the care of cardiogenic shock (CS) patients, including the development of mechanical circulatory support (MCS), the prognosis of these patients continues to be poor. In this context, CS code initiatives arise, based on providing adequate, rapid, and quality care to these patients. In this multidisciplinary document we try to justify the need to implement the SC code, defining its structure/organization, activation criteria, patient flow according to care level, and quality indicators. Our specific purposes are: a) to present the peculiarities of this condition and the lessons of infarction code and previous experiences in CS; b) to detail the structure of the teams, their logistics and the bases for the management of these patients, the choice of the type of MCS, and the moment of its implantation, and c) to address challenges to SC code implementation, including the uniqueness of the pediatric SC code. There is an urgent need to develop protocolized, multidisciplinary, and centralized care in hospitals with a large volume and experience that will minimize inequity in access to the MCS and improve the survival of these patients. Only institutional and structural support from the different administrations will allow optimizing care for CS.
Keywords
This document is endorsed by: the Scientific Associations of the Spanish Society of Cardiology (Interventional Cardiology, Heart Failure, Ischemic Heart Disease, and Acute Cardiovascular Care), the Spanish Society of Pediatric Cardiology and Congenital Heart Disease, the Spanish Society of Anesthesiology, Critical Care and Pain Therapy, the Spanish Society of Cardiovascular and Endovascular Surgery, the Spanish Society of Intensive and Critical Care Medicine and Coronary Units, the Spanish Society of Emergency Medicine, and the Spanish Association of Perfusionists.
Cardiogenic shock (CS) is the most severe form of heart failure, and the 30-day mortality of patients who receive appropriate treatment is between 30% and 50%.1 CS is caused by severe cardiac dysfunction that leads to tissue hypoperfusion and cell hypoxia.2–4 As with any time-dependent process, it can be reversible if the trigger is identified and controlled and measures taken to restore sufficient cardiocirculatory support to maintain optimal systemic perfusion.
The variable effectiveness of treatment can be explained by the different causes, clinical presentation and phenotypes, comorbidities, and the difficulty in identifying reliable risk factors.5 Regarding the etiology, the cardiac dysfunction that leads to CS can be caused by an acute cardiac insult (as in acute coronary syndrome or myocarditis) or decompensation of chronic heart failure (HF).
In 2019, the Society for Cardiovascular Angiography and Intervention (SCAI) established 5 stages: A (at risk of CS), B (beginning CS), C (classic CS), D (deteriorating CS), and E (extremis), easily identifiable based on physical examination, biochemical markers (lactate and degree of metabolic acidosis/base deficit), and hemodynamic parameters,6 and with prognostic implications (mortality reaches 70%-80% in stage E).7 In 2022, some aspects of this were updated, such as cardiac arrest including only those with impaired neurological status, better precision of clinical parameters, and emphasis of the dynamic transition between stages.7 Validation studies support its clinical applicability.4
Successful management of CS is based on the early identification and treatment of the underlying cause, accurate staging, hemodynamic/respiratory stabilization, and the management of multiorgan failure. The aim of this document is to set out the fundamentals to improve management of CS in Spain with protocols that enable quality care adapted to the characteristics of each hospital and each patient. An overview is provided in the executive summary in .
STRUCTURE OF THE CARDIOGENIC SHOCK CODE CARE SYSTEMS AND TEAMSMultiple registry publications have reported experiences and good clinical outcomes with multidisciplinary teams in the setting of a CS code.8–11 The appropriate care of these patients requires organization of health care services: a “hub and spoke” model of care network has been proposed, in which treatment can be delivered according to the patient's needs, in a timely manner, and in the most suitable center. 2,5,7,12 Some of the learning points from the infarct code may be useful when designing this care structure (). As shown by previous local experiences in Spain () and other countries (), the geographical situation and the health care resources of each hospital and health care area should be considered, and the most appropriate treatment should be initiated at the first center, or, if that is not possible, the patient should be referred rapidly to another hospital with expedited transfer.
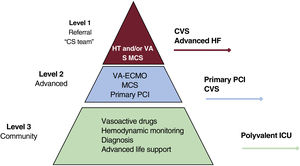
It is essential to designate referral centers in high-volume hospitals, with clearly defined protocols, at the center of a regional system organized by levels of care (table 1 and figure 1).13 The characteristics of the hospitals according to their level of care are described in table 2. Although the most common situation will be that patients who trigger a CS code are identified in the hospital setting, the early identification of those in stages A or B can allow a decision to be made on whether they should be sent directly to a level 2 or 1 center. Either way, level 3 centers play a key role, as the assessment by a critical care specialist in this identifying center (an intensivist, or emergency medicine physician) can avoid treatment delay with early activation of the CS code if the patient deteriorates or does not respond well to the initial treatment. Level 2 centers should have the capacity to implant short-term mechanical circulatory support (MCS) devices. These centers can play a very important role in receiving patients in CS and implanting extracorporeal membrane oxygenation (ECMO). Lastly, level 1 centers (and some level 2 centers with the required structure) should have multidisciplinary teams, whose aims, members, and functions are shown in table 3. The definition of care levels is no simple task. A key factor in level 1 centers is having extensive experience in the use of various MCS devices. In addition, the evidence supports the need for these patients to be managed by specialists with experience and competencies in the care of critically ill cardiovascular patients.14–16 These specialists are also essential to a coordinated approach that allows the rapid evaluation of the patient and activation of the CS code.17,18 Recently, the term “shock doc” has been proposed for specialists with experience in cardiological critical care who are responsible for coordinating decisions and interventions.17
Characteristics of a hierarchical regional organization to enable the cardiogenic shock code
| Categorized interhospital regional network |
| Consensus on selection criteria |
| Capacity for rapid contact between centers |
| Protocol-baswed indication for and type of mechanical circulatory support |
| Protocol-based transfers and transport between centers |
Central illustration. Hospital levels of care for the treatment of cardiogenic shock. CS, cardiogenic shock; CVS, cardiovascular surgery; HF, heart failure; HT, heart transplant; ICU, intensive care unit; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; S, surgery; VA, mid/long-term ventricular assistance; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Characteristics of the different levels of hospital involved in CS management
| Level 3 or community (identification of CS): polyvalent ICU, with no cardiac surgery, primary angioplasty, or MCS |
| Level 2 or advanced (initial CS management): round-the-clock program of primary angioplasty and cardiac surgery. Capacity to implant short/mid-term MCS devices |
| Level 1 or advanced + long-term options (definitive CS treatment): multidisciplinary CS teams, extensive experience in percutaneous and surgical implantation of short-term MCS devices, accredited mid-/long-term MCS or HT programs |
CS, cardiogenic shock; HT, heart transplant; ICU, intensive care unit; MCS, mechanical circulatory support.
Aims of the multidisciplinary cardiogenic shock team, its members and their roles in MCS assessment and choice
| AimsEnsure rapid diagnosisIdentify the specific phenotypeAssign the appropriate level of careMake decisions on interventions and MCSRecognize futility and adopt palliative measuresIdentify candidates for clinical trials | |
| Members | Roles |
| Physicians and nurses from the hospital emergency departments and out-of-hospital emergency medical services | First contact with the patient if not already admittedRisk stratification and initial managementDecision on receiving hospitalTransfers between hospitals with level 1 or 2 support |
| Intensivist/critical care cardiologist/anesthesiologist/cardiovascular surgeon and critical care nurses | Coordinate the processIdentification, stratification, and diagnosisMedical treatmentInvasive hemodynamic monitoringMonitoring, planning and early decision on MCSPostintervention and postoperative monitoringNeurological assessmentRehabilitation and nutritionAppropriate therapeutic/palliative measuresEnd-of-life care/donation |
| Cardiologist specialized in heart failure and transplantation | Medical treatmentLong-term MCS decisionIndications and contraindications for heart transplant |
| Interventional cardiologist and interventional nursing staff | Coronary or structural interventionDecision on early MCS implantationPercutaneous implantation of short-term MCS |
| Surgical block/cardiac and/or vascular surgeon, anesthesiologist, perfusionist, and surgical nurses | Surgical implantation of short- and mid-term MCSHeart transplant/long-term MCSMonitoring of MCS device during its implantation, exchange, or transfer |
MCS, mechanical circulatory support.
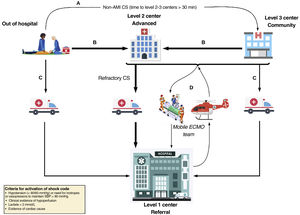
The organization of the CS system needs to include transfers to level 1 centers, MCS implantation in level 2 centers and implantation in level 3 centers by mobile teams from level 1 or 2 (figure 2). Table 4 presents the composition of the mobile teams who must adapt to the regional situations and be available 24hours a day, 7 days a week, with direct telephone contact with the level 2 and 3 hospitals. It is especially important that the cannulating physician is highly experienced in the vascular approach. With the creation of these teams, which can travel to other centers and implant a circulatory support device, mainly ECMO, a survival benefit has been demonstrated in these patients.19,20 The means of transport recommended for distances <400km is by road, and plane is recommended for distances> 600km (table 5). In the case of island transport, the decision should be individualized depending on the distance to be traveled and the weather conditions. Complications may arise in any transfer (table 6).
Patient flow in the cardiogenic shock care network. A: to ensure early stabilization of a patient with CS not caused by acute myocardial infarction (AMI) diagnosed out of hospital, the patient may be transported to the closest level 3 center if transfer to a level 1 or 2 center is in excess of 30minutes longer than to the level 3 center. B: patients with CS diagnosed out of hospital or who are in a level 3 center should be transferred to a level 1 or 2 center depending on the transfer times, especially in the context of acute coronary syndrome. C: patients with CS diagnosed out of hospital or in a level 3 center can be transferred to a level 1 center if they are expected to require complex care. D: activation of the ECMO team; deployment of a mobile unit from the level 1 center to its different referring centers (levels 2 and 3) if implantation of complex mechanical circulatory support is needed to ensure a safe transfer. CS, cardiogenic shock; ECMO: extracorporeal membrane oxygenation; SBP, systolic blood pressure.
Mobile ECMO team, profiles, and roles
| Team member | Profile | Roles |
|---|---|---|
| Team leader | Cardiologist/intensivist/anesthesiologist/cardiovascular surgeon, experienced in ECMO | LeaderCoordination of the teamMedical treatment of the patientCollaborate on cannulation procedure |
| Cannulating physician | Interventional cardiologist/cardiovascular surgeon/critical care specialist* | CannulationSecure cannulas |
| ECMO specialist | Cardiologist/intensivist/anesthesiologist experienced in ECMO. Perfusion nurses or critical care nurses trained in ECMO | ECMO flushingInitiate treatmentEnsure device is functioning correctlyMonitor clotting/blood gases |
| Critical care nurses | Nurses experienced in critically ill patients | Prepare material (checklist)Support during cannulation/instrumentationSupport nursing staff during transport |
ECMO, extracorporeal membrane oxygenation.
Means of transport for transfer of patients with cardiogenic shock and mechanical circulatory support/ECMO
| Ambulance | Helicopter | Plane | |
|---|---|---|---|
| Distance for reasonable time | ≤ 400 km | ≤ 650 km | Any distance |
| Noise | Relatively quiet | Very noisy | Noisy |
| Cost | ++ | +++ | ++++ |
| Weight limits | No limit | Depends on the aircraft and the weather conditions | Variable, depending on the aircraft and the conditions |
| Space for staff and equipment | Sufficient (4-5 members) | Limited (3-5 members) | Variable (≥ 4 members) |
| Setup logistics, securing equipment, and ECMO circuit/patient | Relatively simple | Relatively simple | Variable depending on the equipment and the aircraft |
| Logistics on arrival | Additional transport not required | Hospital heliport or airport. Additional transport may be required | Requires suitable airportAdditional transport required |
| Effect of weather | ++ | ++++ | +++ |
ECMO, extracorporeal membrane oxygenation.
All vehicles must have a) a power supply suitable for ECMO and all other equipment for the duration of transport; b) climate control; c) reliable oxygen supply (in addition to transport cylinders); d) an aspiration system; e) compressed air; f) adequate lighting; and e) adequate space for the necessary staff and equipment.
Complications related to transport of patients on mechanical circulatory support and strategies to minimize them
| Complications | |
| Patient-related | Accidental extubationLow level of sedationHypovolemiaRecirculationArterial ischemiaBleeding |
| Staff-related | Forgetting equipmentLack of staffingCommunication errors |
| Equipment-related | Circuit thrombosisCannula movementDefective materialsElectrical failure/battery failure |
| Transport-related | Malfunction of power sourceLogistical errorsTrafficUnsuitable ambulance |
| Environment-related | Weather conditionsDecompressionFreezing of venous accessHypothermia |
| Strategies to minimize | Clear, accurate, detailed communication of information between all those involvedEnsure the safety of the professionals and that they are familiar with proceduresOfficial referral protocol between hospitalsRegular team training, with simulation if possibleChecklistsPortable ultrasound with cardiac and vascular probes |
Naturally, the first gauge is the very existence of regional multidisciplinary CS care programs (CS code). It is also very important to record the in-hospital mortality rate for CS (patients who died from CS/all patients admitted with CS) and the percentage of patients with CS secondary to an acute coronary syndrome who undergo emergency coronary angiography (< 120minutes). This provides information on the integration between the infarct code network and the CS network. Lastly, the percentage of MCS devices that are registered in the national registry of circulatory and respiratory support devices in Spain (the RENACER Registry) should be recorded. As this is a compulsory registry, it should be 100%.
It is also important to record measures that help prevent CS, primarily those recommended in the infarct code. It is estimated that 1 in every 5 deaths from CS could have been avoided with a time from first medical contact to primary angioplasty within the recommended 90minutes.21 In recent decades, the proportion of cases of CS due to ACS has decreased.5
MECHANICAL CIRCULATORY SUPPORT: TIMING OF IMPLANTATION AND CHOICE OF DEVICEThe types of short-term MCS used in Spain and their contraindications are described in . The current lack of evidence from randomized trials of a benefit from the different MCS systems means that the scientific societies’ recommendations on their indications, the timing of implantation, and the type of device are relatively loose,22 leaving considerable leeway up to the experience of each team. One of the more difficult decisions in the treatment of CS is the timing of MCS implantation and the choice of device. The concept of door-to-treatment time has gained relevance in recent years. Several registries have shown that the more severe the CS at the time of device implantation, the lower the probability of survival.23 Current evidence indicates that timely MCS implantation has a strong effect on prognosis.11,24,25 MCS is particularly indicated, unless futile, in refractory CS (stages D and E). In stages B and C without respiratory failure/hypoxia, a detailed echocardiographic and hemodynamic assessment should be carried out to determine the need for MCS and type of device depending on ventricular function and degree of congestion. In stage C with hypoxemia and in stages D and E, MCS with ECMO combined with intra-aortic balloon counterpulsation or Impella (Abiomed, USA) should be considered.
In the context of patients with CS secondary to acute myocardial infarction, the current recommendations are for MCS implantation prior to revascularization.11,24,25 This approach is associated with a reduced infarct size.26 The results from the Detroit Cardiogenic Shock Initiative suggest something similar, although that study evaluated survival.11 Recently, a meta-analysis including 6700 patients confirmed that mechanical support with Impella prior to angioplasty drastically reduced 30-day mortality.27 This strategy is being validated by the DanGer shock trial, which is currently in the enrolment phase.28 However, the use of ECMO in this situation is less clear, as it can increase left ventricular afterload and oxygen consumption. From the pathophysiological perspective, it is not the ideal support for CS in the initial phase, but progression to a more severe phase of CS means not only pump failure but circulatory and multiorgan failure, in which the high flows that ECMO can deliver, along with a left ventricular unloading device, can play an important role. We are also awaiting the publication of the clinical trials currently underway with ECMO in this context: ExtraCorporeal Life Support in patients with acute myocardial infarction complicated by cardiogenic shock (ECLS-shock),29 EURO-Shock,30 and Assessment of ECMO in Acute Myocardial Infarction Cardiogenic Shock (ANCHOR-NCT04184635).
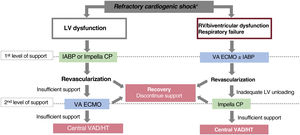
The choice of device in CS not caused by acute myocardial infarction is more complex. The etiology of the clinical presentation and the severity are fundamental to this decision (SCAI classification, biventricular involvement, respiratory status). Assessment of right ventricular function is of great importance.31 In patients with preserved right ventricular function, balloon counterpulsation or an Impella can be enough to provide adequate support in some cases, while ECMO is the device of choice if there is biventricular dysfunction or associated respiratory failure (figure 3).32 The outcomes of ECMO appear to improve with the addition of a left ventricular unloading device,33 although the usefulness of ECMO plus Impella remains to be confirmed in the ongoing clinical trial Randomized trial of Early LV VEnting using impella CP for Recovery in patients with cardiogenic Shock managed with VA-ECMO (REVERSE)34. For patients with isolated right ventricular dysfunction, there are percutaneous continuous flow systems dedicated to right ventricular unloading. One unresolved question is the choice between a counterpulsation balloon and the other percutaneous left ventricular unloading devices. Although the percutaneous unloading devices provide a much superior flow to the counterpulsation balloon, their clinical superiority has not yet been demonstrated, and some studies have described a higher incidence of complications with these devices, either alone35 or combined with venoarterial ECMO.33
Patient selection and choice of device for patients with cardiogenic shock (CS). HT, heart transplant; IABP, intra-aortic counterpulsation balloon pump; LV, left ventricle; RV, right ventricle; VA, venoarterial; VAD, ventricular assist device.
*SBP <90mmHg for more than 30min or inotropes to get SBP> 90mmHg, signs of pulmonary congestion and poor perfusion and at least one of the following: altered mental state, cold clammy skin, oliguria<30mL/h or arterial lactate> 2.0 mmol/L. Refractory CS is CS despite vasopressors/inotropes and appropriate volume replacement.
Cardiorespiratory arrest is a special situation, which obviously carries a different prognosis and treatment protocol. In this emergency situation, there is often not enough information and it is reasonable to use MCS as a bridge to decision-making once the care team has all the necessary information.
PARTICULAR FEATURES OF THE PEDIATRIC CARDIOGENIC SHOCK PROTOCOLThe most common causes of pediatric CS are acute or fulminant myocarditis, decompensated complex congenital heart disease or cardiomyopathy, and myocardial failure after heart surgery, and the most common age of presentation is <1 year.36 The incidence of HF in patients younger than 18 years is estimated at 1 to 7/100 000 and the estimated annual incidence of hospital admission is 14 to 18/100 000.37 Mortality (7%-26%) exceeds 30% when there is associated kidney or liver failure and reaches 50% if ECMO is required.37 CS is treated in pediatric intensive care units that have a pediatric cardiologist. Although Spain has 16 pediatric heart surgery units, not all the autonomous communities have one. If we consider the low incidence of CS and the complexity of its treatment, it seems reasonable to establish common criteria and expedited referral mechanisms to these referral centers. Treatment of pediatric CS often requires MCS.38 The usual short-term MCS in pediatrics is ECMO, and its use, although initially limited to 2 to 3 weeks, has recently been successfully extended to 3 months.39 However, most pediatric hospitals do not have the human and technical resources for ECMO implantation, so there is a need for multidisciplinary teams, comprising surgeons, intensivists, and perfusionists, who can implant on site and transfer the patient to a specialized unit.40 The need for MCS in patients with congenital heart disease is mainly in cases of CS after extracorporeal circulation that need urgent ECMO as a bridge to recovery. Patients with congenital heart disease that has not been surgically repaired are also candidates for MCS, especially univentricular disease with severe decompensation as a bridge to surgery or transplant.39 6Sixty percent of patients requiring MCS have treatment-refractory myocarditis or cardiomyopathy. Short-term MCS is useful as a bridge to recovery or as a bridge to a long-term MCS, but is limited as a bridge to transplant, as the median wait time for emergency transplant is longer than 3 months.41 In Spain, both pulsatile paracorporeal devices (Berlin Heart EXCOR, Berlin-Heart AG, Germany) and continuous paracorporeal devices (Thoratec PediVAS/CentriMag, Thoratec, USA; Maquet Rotaflow, Maquet, Germany) are used as a bridge to heart transplant42 (table 7). The international experience has grown enormously in recent years and includes intracorporeal continuous flow systems for patients of a suitable size, generally older than 12 years and with a weight> 40kg (HeartMate 3, Abbott Labs, USA; Heartware, HeartWare Inc., USA, although Heartware is not currently available).43 Support platforms have been developed that have helped improve outcomes and reduce thrombotic complications.44 Currently, 40% of patients younger than 18 years survive to transplant with an MCS device.41 Survival is similar for patients who undergo this electively or as an emergency with long-term ventricular assistance, but is lower for patients on ECMO, those younger than 1 year, and patients with congenital heart disease.41 The special characteristics of children with CS require treatment in special pediatric HF and transplant units.
Pediatric mechanical circulatory support devices
| Device | Venoarterial ECMO | Continuous flow paracorporeal support | Pulsatile flow paracorporeal support | Continuous flow intracorporal support | Total artificial heart |
|---|---|---|---|---|---|
| General points | |||||
| Experience | A lot | Moderate | Abundant | Little | Anecdotal |
| Duration of support | Short (2-3 weeks) | Medium (3-6 weeks) | Long (months) | Months/Years | Months/Years |
| Patient mobilization | No | Occasionally | Yes | Yes | Yes |
| Technical details | |||||
| Blood flow | Continuous | Continuous | Pulsatile | Continuous | Pulsatile |
| Respiratory support | Yes | No (possible) | No | No | No |
| Circulatory support | Biventricular | Univentricular or biventricular | Univentricular or biventricular | Univentricular or biventricular | Biventricular |
| Cannulation | Vascular | Cardiac | Cardiac | Intracardiac | Heart replacement |
| Ventricular unloading | Incomplete | Almost complete | Complete | Complete | Complete |
| Anticoagulation | Yes | Yes | Yes | Yes | Yes |
| Antiplatelet therapy | No | Yes | Yes | Yes | Yes |
| Indications | Bridge to recoveryBridge to decisionBridge to support | Bridge to transplantBridge to recovery (late) | Bridge to transplant | Bridge to transplantBridge to destination | Bridge to destination Bridge to transplant |
| Devices | Various | Thoratec PediVASThoratec CentriMag (Thoratec, USA)Maquet Rotaflow (Maquet, Germany) | Berlin Heart EXCOR (Berlin-Heart AG; Germany) | HeartMate3 (Abbott Labs, USA)Heartware (withdrawn) (HeartWare Inc, USA) | SynCardia (Syncardia Systems, USA) |
ECMO, extracorporeal membrane oxygenation.
The CS code represents an organizational challenge for hospitals and between-hospital transport systems. This is an inherent part of structuring a new care circuit that involves changes in patient flow, with an expected increase in demand in some centers and reduced demand in others. One of the main obstacles in the proper, successful implementation of the CS code is the individual interests of the various people and hospitals involved. Implementation of the CS code can face several barriers: among them, that hospitals not selected to house the multidisciplinary coordination team may not understand the decision, in addition to a lack of financial resources for establishing the mobile teams. It is therefore absolutely essential that all those involved work for the common good and collaborate actively in developing the protocol and reach consensus on the criteria for transfer. The availability of material resources and staffing are also essential for the success of such an initiative. Centers anticipating increased patient flow must have the option to increase bed availability (especially in critical care units dedicated to these patients) and the availability of staff, both medical and specialized nursing, depending on the requirements. In addition, the budget for devices and procedures required in this clinical context must be considered. Another crucial aspect for the proper functioning of this type of circuit is to have a robust interhospital transport system. In the case of the CS code it is essential, as treatment times are crucial and the staff in charge of the transfers must have a high level of training and specialization. Hiring and ongoing training of staff, with special emphasis on clinical simulation,45 are essential factors for the success of the program, and much more so if the plan is for remote implantation of MCS devices by the interhospital transfer system staff. Another potential limiting factor is saturation of critical care units in the referral centers. Patients with CS who survive the first few hours have long hospital stays, with a high incidence of complications and need for invasive procedures.46 As occurred with the infarct code in some autonomous communities, certain measures can be implemented to avoid saturation of high-complexity referral centers, such as a consensus on certain conditions for returning patients to lower-complexity hospitals if they reach a certain level of stability, especially if it is decided that they are not candidates for advanced treatment. Similarly, decisions on appropriate level of treatment must be made with the involvement of the multidisciplinary team to avoid futile interventions and unnecessary stays in patients with multiple complications and poor short-term prognosis, a common situation in this context. Lastly, in irreversible situations where support measures are ineffective, we must consider the option of organ or tissue donation.
CONCLUSIONSDespite advances in MCS devices, the prognosis of CS has a wide margin for improvement. This is largely due to the fragmentation of care, nonuniformity of management, and a non–protocol-based approach. Numerous observational registries support the establishment of a centralized, integrative, multidisciplinary CS code. The CS code is feasible and can improve survival in these patients, allowing early diagnosis and prompt MCS implantation, with appropriate revascularization strategies and timing. Institutional support is essential for the success of this initiative.
FUNDINGThis article did not receive funding and the authors have not received any payment for their participation.
AUTHORS’ CONTRIBUTIONSAll the authors have contributed substantial intellectual content and have approved the final text of the main document and the appendices. J. Díaz and F. Fernández-Avilés contributed equally to this article.
CONFLICTS OF INTERESTJ.M. de la Torre-Hernández: consultancy honoraria from Medtronic, Boston Science, Abbott; payment or honoraria for conferences, presentations, speaker workshops, writing manuscripts and educational events from Medtronic, Abbott, Boston Science; support to attend meetings and/or travel from Biotronik, Abbott; participation in a supervisory board on data security and in an advisory board from Medtronic and Philips. A. Sionis Green: honoraria for consulting/conferences/presentations from Amgen, Daiichi-Sankyo, Novartis, Sanofi, and Servier. J.M. Barrio: honoraria for conferences/presentations from Edwards Lifesciences. A. Uribarri: honoraria for consulting/conferences/presentations from Abbott. M. Monteagudo: honoraria as consultant for Abiomed. The other authors have no conflicts of interest.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.10.014s