Myotonic dystrophy type 1 (DM1) or Steinert disease is a multisystem disease with a genetic basis, caused by the pathological expansion of the cytosine-thymine-guanine trinucleotide repeat in the 3’ untranslated region of the myotonic dystrophy protein-kinase gene on chromosome 19.1
The resulting transcription of expanded, mutated mRNA forms focal accumulations in the cell nucleus, leading to hijacking and altered regulation of several proteins associated with the splicing of pre-mRNA molecules. This in turn affects the expression of several genes and causes the pathophysiological and clinical consequences of DM1, such as myotonia.
Myotonia is a skeletal muscle disorder consisting of hyperexcitability of the sarcolemma due to a reduction in the number and function of chloride channels, which leads to muscular rigidity and delayed relaxation after contraction. In DM1, the abnormal expression of the chloride channel produces loss of its function through a dominant-negative effect,2 which causes myotonia.
Mexiletine has an antimyotonic effect through various mechanisms, one of which is by blocking the sodium current in skeletal muscle (NaV1.4), which reduces the probability of repetitive action potentials.3 The class 1b antiarrhythmic effect blocking voltage-gated sodium channels reduces the conduction velocity and increases the duration of the action potential. Its potential effects on the electrocardiogram (ECG) are increased QRS duration (especially at high heart rates) and shortening of the corrected QT interval.
In DM1, intraventricular conduction defects commonly occur through various mechanisms, which affect 30% to 70% of patients and which can cause atrioventricular block or facilitate ventricular tachycardias through a bundle-branch reentry mechanism, both of which may cause sudden cardiac death. Therefore, in patients with DM1, there are questions about the cardiovascular safety of mexiletine and other sodium blockers, which have led to some authors suggesting it should be avoided.4
There is some evidence of a benefit with mexiletine used for short periods in improving myotonia in patients with DM1 without increased cardiac risk or ECG changes.3,5 The long-term risk-benefit balance will not be well-established until the results of a phase 3 clinical trial, currently in the design stage, become available (Clinicaltrials.gov NCT04700046). The available data indicate that mexiletine (400mg daily) is safe in selected patients and does not increase the incidence of electrocardiographic changes or bradyarrhythmic complications.6
The aim of our study was to present the results of a retrospective review of the use of mexiletine as a drug authorized for “compassionate use”, having obtained prior informed consent, in a cohort of patients with DM1 at a tertiary referral center.
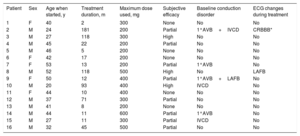
During the period 2000 to 2020, 16 patients were identified (69% men) with DM1 treated with mexiletine, with an age at DM1 diagnosis of 29±12 (range, 7-53) years (table 1). The median cytosine-thymine-guanine triplet repeat size was 500 [51-850].
Key patient characteristics
| Patient | Sex | Age when started, y | Treatment duration, m | Maximum dose used, mg | Subjective efficacy | Baseline conduction disorder | ECG changes during treatment |
|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 2 | 300 | None | No | No |
| 2 | M | 24 | 181 | 200 | Partial | 1°AVB+IVCD | CRBBB* |
| 3 | M | 27 | 118 | 300 | High | No | No |
| 4 | M | 45 | 22 | 200 | Partial | No | No |
| 5 | M | 46 | 5 | 200 | None | No | No |
| 6 | F | 42 | 17 | 200 | None | No | No |
| 7 | F | 53 | 13 | 200 | Partial | 1°AVB | No |
| 8 | M | 52 | 118 | 500 | High | No | LAFB |
| 9 | F | 50 | 12 | 400 | Partial | 1°AVB+LAFB | No |
| 10 | M | 20 | 93 | 400 | High | IVCD | No |
| 11 | F | 44 | 10 | 400 | None | No | No |
| 12 | M | 37 | 71 | 300 | Partial | No | No |
| 13 | M | 41 | 8 | 200 | None | No | No |
| 14 | M | 44 | 11 | 600 | Partial | 1°AVB | No |
| 15 | M | 27 | 11 | 300 | Partial | IVCD | No |
| 16 | M | 32 | 45 | 500 | Partial | No | No |
1°AVB, first-degree atrioventricular block; CRBBB, complete right bundle branch block; ECG, electrocardiogram; F, female; IVCD, nonspecific interventricular conduction defect with QRS interval>110 ms (no criteria for left or right bundle branch block); LAFB, left anterior fascicular block; M, male.
The age at starting mexiletine was 39±10 (20-53) years. No patient had a history of syncope or cardiac arrest or a pacemaker. Systolic function was normal in all except 1 patient who had mild dysfunction.
Baseline ECG prior to treatment showed sinus rhythm in all patients, 4 (25%) cases of first-degree atrioventricular block, one of which was associated with a nonspecific intraventricular conduction defect, and the other with left anterior fascicular block; 2 (12.5%) patients had an isolated nonspecific intraventricular conduction defect. The corrected QT interval was normal in all patients.
The mexiletine dose used was 325 (200-600) mg/d, and the mean treatment duration was 46 [2-181] months. According to subjective evaluation as per normal practice, mexiletine was considered ineffective in controlling myotonia in 31%, partially effective temporarily (reason for stopping) in 50%, and very effective in 19%, who remain on this treatment to date. In none of the patients was it stopped due to cardiac complications; 25% had gastrointestinal upset that did not require medication withdrawal.
During treatment there were no significant cardiovascular events such as syncope, pacemaker requirement, atrioventricular block, bundle-branch re-entry ventricular tachycardia, or sudden cardiac death. On serial ECGs, the onset of left anterior fascicular block was documented in 1 patient, and in another patient, right bundle branch block, which was initially rate-dependent and then later became established during treatment.
After stopping treatment, during follow-up of the cohort, 2 pacemakers were implanted: 1 for symptomatic sinus node dysfunction and another in an asymptomatic patient with onset of first-degree atrioventricular block and complete bundle branch block with pathological prolongation of the HV interval on the electrophysiology study done as standard. There were 4 deaths (25%), 2 due to stroke, 1 due to pneumonia, and 1 due to severe respiratory failure.
In conclusion, the long-term use of mexiletine to improve myotonia in patients with DM1 without severe conduction defects on baseline ECG, even at high doses, does not appear to be associated with an increased cardiac risk and shows evidence of modest effectiveness. The limitations inherent to the retrospective nature of this cohort study must be taken into account when evaluating the results, which should be supported with a prospective study.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSR. Salguero-Bodes: study conception, data collection, analysis, and manuscript writing. A. Ruiz-Curiel: data collection, analysis, manuscript review. Remaining authors: participation in conception and critical review of the manuscript.
CONFLICTS OF INTERESTThere are no potential conflicts of interest.