Left atrial appendage (LAA) closure with the WATCHMAN device (Boston Scientific; Minnesota, United States) is as effective as warfarin in preventing embolisms in patients with nonvalvular atrial fibrillation (NVAF) and is associated with a comparative reduction in bleeding events and cardiovascular mortality.1 This procedure is a feasible alternative to oral anticoagulant (OAC) therapy for patients with a high bleeding risk.
Residual leakage around the device is an important limitation in LAA closure. The causes of this event include variability of the LAA ostium shape, underestimation of its size, device migration, and difficulty in covering several lobes.2
The optimal treatment for peridevice leakage following WATCHMAN implantation is uncertain. In patients who have undergone LAA surgical ligation, embolism rates are higher in those with residual communications than in those with complete closure.3 It is accepted by consensus that leaks <5mm wide are not significant, and they are not considered a procedure failure.4 Treatment for leaks>5mm in size is controversial. In contrast to the recommendation to maintain OAC indefinitely, some authors suggest percutaneous closure as a viable option, considering the patients’ bleeding risk and the development of technical aids such as microwave use in transesophageal echocardiography (TEE).5 Experience with this type of intervention is scarce and limited to small case series.2 Here, we report on the first 2 cases in Spain of percutaneous closure of major peridevice leaks (>5mm) associated with WATCHMAN.
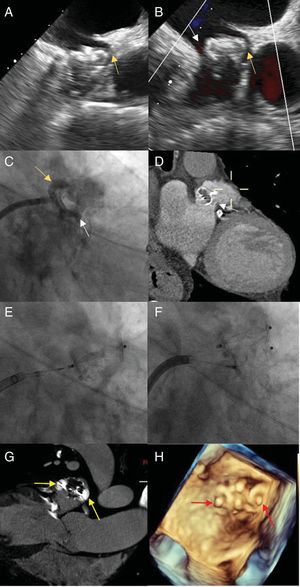
The first case was an 85-year-old man with permanent NVAF and indefinite OAC, a history of lower gastrointestinal tract bleeding, hypovolemic shock, and hemorrhagic stroke, and a CHA2DS2-VASc score of 6 and HAS-BLED score of 5. Implantation of a 24mm WATCHMAN device was carried out, with adequate intraoperative compression and detection of an anterosuperior leak <3mm wide (figure 1A, arrow) that was treated conservatively. After 1 year with no incidents, the patient experienced an ischemic stroke in the left frontal territory. TEE showed proximal migration of the device and 2 leaks >5mm wide, one in an anterosuperior position (figures 1B,C, yellow arrow) and the other inferoposterior (figure 1B-D, white arrow). Possible causal mechanisms include poor initial fixation or posterior LAA dilation due to the initially incomplete closure, as occurs in cases of incomplete percutaneous closure. Because of the patient's contraindications for restarting OAC, percutaneous closure was decided. An 8.5-Fr Medium Curl Agilis sheath (Abbott; Illinois, United States) was placed in the left atrium, a 5-Fr multipurpose guide catheter and 0.035-inch straight hydrophilic guide (Terumo Europe; Leuven, Belgium) were advanced to cross the 2 leaks, and 2 12-mm Amplatzer Vascular Plug II devices (Abbott; Illinois, United States) were successfully implanted (Figure 1E-H).
A: transesophageal echocardiography image showing an anterosuperior leak <3mm wide (yellow arrow) following the first procedure. B: transesophageal echocardiography image depicting an anterosuperior leak (yellow arrow) and inferoposterior leak (white arrow) after the patient experienced a stroke. C: fluoroscopy image showing the anterosuperior (yellow arrow) and inferoposterior (white arrow) paravalvular leak. D: computed tomography image of the inferoposterior leak (white arrow). E: fluoroscopy image depicting anterosuperior implantation of a 12-mm Amplatzer Vascular Plug II. F: fluoroscopy image showing posteroinferior implantation of a 12-mm Amplatzer Vascular Plug II. G: computed tomography image of the final position of the devices (yellow arrows). H: 3-dimensional ultrasound image of the final position of the devices (red arrows).
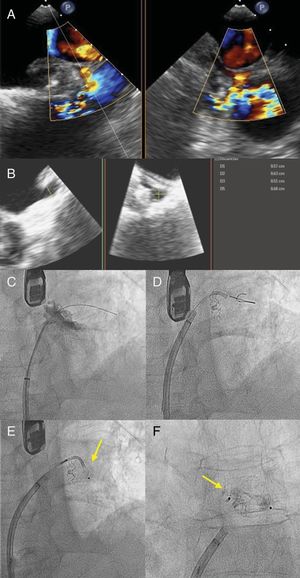
The second case was a 72-year-old man with a history of paroxysmal NVAF, CHA2DS2-VASc score of 3 and HAS-BLED score of 3, and a history of cerebral arteriovenous malformation that contraindicated OAC therapy. A 21-mm WATCHMAN device was implanted, with adequate compression. At 1 year following the procedure, TEE showed an anterosuperior leak >5mm in size (figure 2A,B), and percutaneous closure was decided. An 8.5-Fr Medium Curl Agilis sheath was positioned in the left atrium. Sufficient support was not attained with a 0.035-inch straight hydrophilic guide; hence, a 0.014-inch Sion angioplasty guide (Asahi Intecc; Amsterdam, The Netherlands) was used, and telescoped on top of it, a 150-cm Glidecath 4-Fr MP (Terumo Europe; Leuven, Belgium) and a Finecross microcatheter (Terumo Europe). With this additional support, the leak was crossed and an 8-mm Amplatzer Vascular Plug II device was released (figure 2C-F).
Both patients were discharged the next day with dual antiplatelet therapy (aspirin+ clopidogrel) for 3 months, to continue with aspirin 100mg/d indefinitely. Neither patient experienced a new embolic or hemorrhagic event after discontinuing clopidogrel and during a follow-up of 4 and 14 months, respectively.
In the absence of additional experience, the initial safety and efficacy2 results suggest that percutaneous closure of these types of defect could be considered within a more general preventive strategy, particularly since LAA closure is most often indicated in patients with OAC-related bleeding problems.
CONFLICTS OF INTERESTA. Pérez-de Prado is a clinical supervisor for Boston Scientific.