Pulmonary embolism (PE) is the leading cause of hospital death and the third most frequent cause of cardiovascular mortality. Traditionally, treatment options have included anticoagulation, thrombolysis, or surgery; however, catheter-directed interventions (CDI), including catheter-directed thrombolysis and aspiration thrombectomy, have been developed for patients with intermediate- or high-risk PE. These techniques can rapidly improve right ventricular function, hemodynamic status, and mortality in some patients, although there is a lack of evidence from randomized controlled trials. This document, prepared by the Interventional Cardiology Association, the Association of Ischemic Heart Disease and Acute Cardiovascular Care, and the Working Group on Pulmonary Hypertension of the Spanish Society of Cardiology (SEC), reviews the current recommendations and available evidence on the management of PE. It emphasizes the importance of rapid response teams, risk stratification, and early patient monitoring in identifying candidates for reperfusion. Based on existing clinical evidence on CDI, the document discusses various clinical scenarios and provides guidance on patient selection, particularly in situations of uncertainty due to insufficient evidence. Lastly, it describes periprocedural support, highlighting the necessary multidisciplinary approach to improve outcomes and reduce morbidity and mortality in patients with PE.
Keywords
Pulmonary embolism (PE), the third most frequent thrombotic syndrome after myocardial infarction and stroke, is the leading preventable cause of death in hospitalized patients. In PE, acute obstruction of the pulmonary arteries causes right ventricular pressure overload, which, if left untreated, can culminate in shock and death. New catheter-directed intervention (CDI) techniques are revolutionizing the treatment of severe PE as an alternative to systemic thrombolysis (ST).
PE necessitates a multidisciplinary approach that relies on a cardiologist for diagnosis, risk stratification (electrocardiogram, echocardiogram), hemodynamic stabilization (cardiac intensive care units [CICUs], extracorporeal membrane oxygenation [ECMO]), CDI, and follow-up (chronic thromboembolic pulmonary disease [CTEPD] and balloon pulmonary angioplasty).
The present document reviews the current evidence-based recommendations and provides the opinions of an expert panel on areas lacking sufficient evidence (table 1). The document focuses on the stratification and risk/benefit assessment of candidates for CDI but also makes recommendations on periprocedural support. The emphasis is on promoting the implementation of PE response teams (PERTs), with the corresponding admission of patients to specialized units for the use of advanced therapies that can reduce PE-related morbidity and mortality.
Summary of the definitions and recommendations of the expert panel on situations with scarce evidence
| Definition of reperfusion | Reperfusion is defined as targeted therapy to rapidly restore pulmonary flow by reducing the thrombus burden that is causing RV pressure overload, typically in situations of obstructive shockManagement comprises pharmacological (ST), percutaneous (CDI), and surgical (SE) treatment |
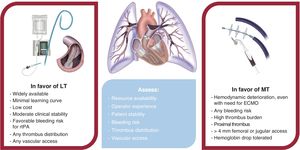
| CDI in HR-PE | In patients with HR-PE who do not undergo ST due to contraindications or high bleeding risk, CDI is recommended if it can be performed within 60 min or within 90 min if transfer is requiredIn the case of CDI in HR-PE, MT is recommended over LTIn the case of CDI in HR-PE, LT can be used if MT is not available and the patient exhibits sufficient stability to permit therapy completion (eg, persistent hypotension) |
| Elective CDI in IHR-PE | The initial management of patients with IHR-PE includes monitoring in intensive or intermediate care for 24-48 h and anticoagulationIn this period, for selected patients with IHR-PE, elective CDI should be considered if patients have several poor prognostic factors to prevent clinical or hemodynamic deterioration. This decision should be made in the first 24 hPatients with IHR-PE and clinical or hemodynamic deterioration according to ESC guidelines should undergo CDI over ST due to its better safety profile |
| MT completion criteria | There are no defined criteria indicating MT completionAn attempted removal of all thrombus burden is not recommended to avoid prolonging the procedure and potential mechanical complicationsThe objective should be an improved clinical situation in patients with IHR-PE and hemodynamic stability in patients with HR-PE. Objective parameters are a reduction in vasoactive support, increase in SBP, decrease in HR, improvement in the PAFI, or an improved venous saturationOther completion criteria would be extraction of all thrombi in the proximal arteries, a chronic or well-established thrombus, an anatomical limitation that limits thrombus access, or an unfavorable balance between thrombi and blood (extraction > 400-500 mL) |
| Requirements of the expert center in PE | PERT available 24 h/dCDI (ideally both LT and MT) available 24 h/dECMO available 24 h/dThe need for cardiac surgery is controversial, given that SE as a reperfusion modality is in disuse, although it may be useful for thrombus-in-transit across a patent foramen ovale, ECMO, or, together with vascular surgery, in the resolution of complicationsCenters without cardiothoracic surgery should have the support of a referral center that can treat emergency cases (in a network) |
| Times | After PE diagnosis, the initial therapeutic strategy should be established in the first 30-60 min (comprising risk stratification and PERT consultation)In IHR-PE, monitoring in an intermediate or intensive care unit for the first 48 h after diagnosis is recommendedIn IHR-PE, evaluation of poor prognostic factors (table 2) is recommended in the first 6 h after admission to assess candidates for elective CDIIf CDI is selected in IHR-PE, it should be performed within 24 h of the decisionIf CDI is selected in HR-PE, it should be performed within 4 h of the decisionAfter reperfusion, monitoring is recommended for 24 h in IHR-PE and for 48 h in HR-PE |
| Reperfusion success in HR-PE | Reperfusion is considered successful if a SBP < 90 mmHg is achieved without vasopressorsThis assessment should be performed 2-4 h after ST administration or at CDI completion |
CDI, catheter-directed intervention; ECMO, extracorporeal membrane oxygenation; ESC, European Society of Cardiology; HR, heart rate; HR-PE, high-risk pulmonary embolism; IHR-PE, intermediate–high-risk pulmonary embolism; LT, local thrombolysis; MT, mechanical thrombectomy; PAFI, ratio of arterial oxygen pressure to inspired oxygen fraction; PERT, pulmonary embolism response team; SBP, systolic blood pressure; SE, surgical embolectomy; ST, systemic thrombolysis.
PERTs for the management and treatment of high-risk PE (HR-PE) and intermediate–high-risk PE (IHR-PE) were first introduced in the United States in 2012 and currently have a IIa level of recommendation in European guidelines (level of evidence C).8
Comprising different specialists (cardiology, pneumology, internal medicine, emergency medicine, hematology, vascular surgery, anesthesiology, intensive care, cardiac surgery, and interventional radiology), the teams meet to assess patients from multiple perspectives, agree on individualized treatment plans, and activate local resources or arrange patient transfers to referral centers to ensure the necessary level of care.9
Although there is variability in PERT organization, activation frequency, composition (which may involve a hospital network), and available resources, their creation, development, and periodic auditing are fundamental aspects, together with preferential access to key clinical departments (emergency care, echocardiography, radiology, intensive care, percutaneous interventions, and cardiothoracic surgery).9 Evidence indicates that centers with higher volumes of patients with PE have lower 30-day mortality and that PERT activation is associated with lower mortality and readmission rates.10 In addition, PERT implementation is associated with higher use of reperfusion and CDI.11–14 Cardiology is crucial to the management of PE and contributes to all its diagnostic and therapeutic phases. The participation of cardiology in PERTs is vital for the treatment of obstructive shock due to right ventricular failure. In addition, cardiologists often work in decision-making teams (Heart Team), with roles in cardiogenic shock, transplantation, and ischemic and valvular heart disease interventions, as well as in evaluating the risks and benefits of alternative treatments.
INITIAL STRATEGY AND RISK STRATIFICATIONRisk stratificationAccording to European guidelines,8 patients are classified based on their hemodynamic stability, clinical score (PE severity index [PESI] or simplified PESI [sPESI]), right ventricular (RV) dysfunction or dilatation, and biomarkers (table 2) into the following categories: a) HR-PE: defined as patients with RV involvement on imaging (echocardiography or computed tomography), elevated biomarkers (troponin, B-type natriuretic peptide [proBNP]), and hemodynamic instability (divided into 3 subcategories: persistent hypotension, obstructive shock, and cardiac arrest)8; about 4% of patients have HR-PE, with short-term mortality of up to 27% within the first 24hours and up to 49% within 72hours; b) intermediate-risk PE: in contrast to HR-PE, these patients are hemodynamically stable; this category is itself subdivided into 2 categories: IHR-PE, characterized by the presence of RV involvement on imaging and elevation in any biomarker; and low–intermediate-risk PE, characterized by the presence of 1 or none of the previous criteria (but PESI III-V or sPESI ≥ 1); this group represents 55% of patients; although most improve with anticoagulation, the IHR-PE subgroup should be closely monitored due to the risk of clinical, respiratory, or hemodynamic deterioration; and c) low-risk PE: these patients are hemodynamically stable, with a PESI I-II or sPESI score of 0 and without RV involvement on imaging or biomarker elevation; they comprise about 40% of all patients with PE.
Clinical, imaging, and laboratory indicators of severity in patients with intermediate–high-risk PEa
| Clinical | Imaging | Laboratory |
|---|---|---|
| SyncopebHR ≥ 110 bpmbSBP <100 mmHgbArterial saturation <90%Respiratory rate > 30 bpmAgeMale sexCancerChronic heart failureChronic pulmonary diseaseTemperature <36°CAltered mental status | RV/LV ratio > 1cTAPSE <16 mmInferior cava dilatationTricuspid annular peak systolic velocity (< 9.5 cm/s)VTI in RVOT <9.5 cm60/60 sign (pulmonary acceleration time <60 ms with PASP <60 mmHg) | Elevated troponincLactate <2 mmol/Lb (another valid cutoff point is > 3.3 mmol/L)NT-proBNP > 600 pg/mL |
| Scales |
|---|
| BOVA score.2 Includes HR, SBP, biomarkers, and RV dysfunction on echocardiography. The extended BOVA adds lactate and permits identification of patients with a 24% risk of hemodynamic deterioration at 7 d |
| TELOS scale.3 Includes, in addition to RV dysfunction and troponin, elevated lactate; these patients have a 21.1% risk of death or hemodynamic collapse at 7 d |
| SHIELD scale.4 Includes a shock index (HR/SBP) ≥ 1, hypoxemia, elevated lactate, and signs of RV dysfunction (biomarkers or imaging markers) |
| FAST score.5 Includes 3 parameters: elevated cardiac troponin, syncope, and HR ≥ 100 |
| NEWS2 scale.6 Is advised for monitoring the detection of clinical deterioration and the need for reperfusion and has been recommended in a European consensus document for use in IHR-PE. It includes 6 parameters: RR, oxygen saturation, temperature, SBP, HR, and level of consciousness |
| Additive score from Bangalore et al.7 With clinical and imaging parameters, this scale can predict normotensive shock (SBP ≥ 90 with a cardiac index <2.2 L/min/m2) in patients with IHR-PE |
| PESI8. Class I: ≤ 65 points; Class II: 66-85 points; Class III: 86-105 points; Class IV: 106-125 points; Class V: > 125 points. Scoring: age in years, 1 point for each year; male sex, 10; cancer, 30; chronic heart failure, 10; chronic pulmonary disease, 10; HR ≥ 110 bpm, 20; SBP <100 mmHg, 30; RR > 30 bpm, 20; temperature <36°C, 20; altered mental status, 60; arterial saturation <90%, 20 |
| sPESI8. 1 point if any of the following criteria are met: age > 80 y, cancer, chronic heart failure, SBP <100 mmHg, arterial saturation <90% |
HR, heart rate; IHR-PE, intermediate–high-risk pulmonary embolism; LV, left ventricular; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PASP, pulmonary artery systolic pressure; RR, respiratory rate; RV, right ventricular; RVOT, right ventricular outflow tract; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion; VTI, velocity-time integral.
In recent decades, efforts have been made to identify a subgroup of IHR-PE patients with short-term risk of hemodynamic deterioration. Although the guideline-recommended therapy is anticoagulation, for patients with a risk of death ≥ 10% according to the various scales and markers, this group of experts recommends considering elective CDI because this technique is associated with ≤3% mortality in various studies, mainly observational.15–20 To identify this subgroup, we propose a series of markers and prognostic scales based on parameters detectable at admission (table 2).
Monitoring and place of admissionPatients with low-intermediate risk can be admitted to a standard hospital floor. Those with IHR-PE require close monitoring to detect early signs of deterioration and should therefore be admitted to an intermediate care unit or CICU. Patients at high risk should be admitted to a CICU.
MANAGEMENT OF PULMONARY EMBOLISMAnticoagulationAnticoagulation is the cornerstone of PE treatment. Treatment with low-molecular-weight heparin or fondaparinux is recommended over unfractionated heparin (UFH) because they more rapidly reach therapeutic concentrations and are associated with a lower risk of heparin-induced thrombocytopenia. A meta-analysis showed that patients with PE initially treated with low-molecular-weight heparin exhibited fewer thrombotic complications, a better safety profile, and lower mortality vs UFH.21 UFH is the drug of choice for patients with chronic kidney disease (clearance <30mL/min), high bleeding risk, morbid obesity, high risk of hemodynamic deterioration, or those who are candidates for CDI. In patients with an absolute contraindication, a vena cava filter can be considered ().
ReperfusionReperfusion is defined as targeted therapy to rapidly restore pulmonary flow by reducing thrombus burden and relieving RV pressure overload, typically in situations of obstructive shock (table 1). Although we report the main international recommendations in , the present document diverges from the indications in the European guidelines and summarizes the most common treatments, as follows.
Systemic thrombolysisThe guidelines recommend reperfusion therapy with ST in patients with HR-PE and as rescue therapy in patients with IHR-PE who exhibit hemodynamic deterioration with anticoagulation. This recommendation was derived from older studies that included both patients with HR-PE and IHR-PE and from meta-analyses of these studies (level of evidence B).8,22
The drug of choice is recombinant tissue-type plasminogen activator (rtPA) alteplase, with streptokinase and urokinase as alternatives. The alteplase dose is a 100-mg infusion over 2hours. In patients with very severe disease, it can be administered in a bolus of 0.6mg/kg every 15minutes (maximum, 50mg). If the patient's weight is <65kg, the maximum dose is 1.5mg/kg. Contraindications are summarized in . There are low-dose ST dosage schedules (typically 0.6mg/kg rtPA, with a maximum dose of 50mg) that are associated with a moderate bleeding risk, between that of anticoagulation and full-dose ST, which are being prospectively evaluated in the PEITHO-3 trial.23,24
UFH can be administered during thrombolysis with alteplase but should be suspended if coagulopathy develops, then restarted once the activated partial thromboplastin time is <2seconds and fibrinogen is >100mg/dL. If low-molecular-weight heparin has been administered, the UFH perfusion should not be restarted until 12hours after the last heparin dose.
The criteria for thrombolysis failure25 are persistence or worsening of the hemodynamic instability 2 to 4hours after its administration.1 In this case, other rescue reperfusion therapies should be considered, such as mechanical thrombectomy (MT).
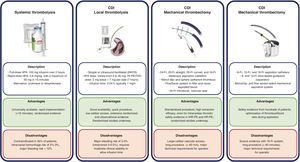
Catheter-directed interventionThe use of percutaneous techniques for the management of PE has grown significantly in the last 10 years.14,26,27 The techniques are divided into 2 groups—local thrombolysis (LT) and MT—and are summarized in figure 1.
In both groups, ultrasound-guided puncture is essential for optimizing safety and ruling out venous thrombosis. Navigating to the pulmonary artery is recommended using a Swan-Ganz or pigtail catheter for a central pass through the tricuspid valve. Pressures should be recorded, including those of the right atrium (preload score) and pulmonary artery, in addition to the initial and final saturation (or pre- and postprocedure values in LT). Pulmonary artery angiography can be performed to confirm the diagnosis and assess the anatomy. An arterial line can be considered for gasometry and invasive blood pressure monitoring, particularly in unstable patients.
Local or intrapulmonary thrombolysisST, which is the standard treatment for HR-PE, is underused, even in the absence of contraindications, due to its high associated bleeding risk (>10% rate of major bleeding, including 2%-3% risk of intracranial bleeding). Pulmonary LT, which permits much lower thrombolytic doses and longer intervals between infusions, became an option due to the simplicity of the technique and the lower bleeding rate, which make it viable even in patients with relative contraindications for ST.28
Devices. LT can be administered using a multiside hole catheter, such as a pigtail catheter, or a perfusion catheter, such as the Cragg-McNamara (Medtronic, United States), which is a multiside hole catheter designed for peripheral thrombolysis and used to treat PE.24 The Bashir catheter (Thrombolex, United States) features a distal nitinol basket that expands within the thrombus to fracture it while thrombolytics are administered at low doses. The EKOS Endovascular System catheter (Boston Scientific, United States) is a thrombolytic infusion system that incorporates transducers that generate high-frequency and low-energy ultrasonic waves to cavitate the thrombus and increase the surface area for the thrombolytic agent.29 This catheter is supported by a higher level of evidence (randomized clinical trials) than its competitors, even though there is insufficient evidence of superiority over conventional catheters.24
Technical details and literature evidence. LT is a technically simple and widely available procedure. One or 2 catheters can be positioned in the main pulmonary artery or in both branches (lower lobes in the case of the EKOS system).18 Although several thrombolytic agents have been used, rtPA is the thrombolytic of choice. Various dose and infusion time regimens have been used (absolute dose from 8 to 24mg administered for between 4 and 24hours),29–32 and all have been associated with improvements in RV overload parameters vs baseline.32 The ideal dose is unclear, and so it may be reasonable to use higher doses for a greater thrombus burden and lower doses for a higher bleeding risk.32 The ongoing HI-PEITHO trialis analyzing whether ultrasound-facilitated LT is superior to anticoagulation in IHR-PE.33
The hemodynamic study and angiography can be repeated within the first 24hours and the system can be withdrawn using compression bandages. It is very rarely necessary to prolong the thrombolytic infusion. UFH administration (300-600 IU/h with previous bolus) is recommended before a procedure. The therapy should be maintained for up to 4hours after LT completion, with an activated partial thromboplastin time <60seconds or an activated clotting time <200seconds.
The ideal candidate characteristics, precautions, and contraindications for these techniques are summarized in table 3 and the evidence on LT is shown in table 4.
Characteristics of patients with pulmonary embolism who are candidates for local or intrapulmonary thrombolysis
| Good candidates | ||
|---|---|---|
| HR-PE | IHR-PE (rescue) | IHR-PE (elective) |
| • Relative contraindication for ST• Absolute contraindication for ST if there are no other treatment options• As long as a sufficient delay can be allowed for the LT to act (persistent hypotension category) | • Clinical or hemodynamic deterioration according to ESC guidelines with anticoagulation, as an alternative to ST due to its better safety profile | • In selected patients with IHR-PE, consider elective CDI if several poor prognostic factors (table 2) are present to prevent clinical or hemodynamic deterioration• Without absolute contraindication for ST (adjusting dose and duration based on bleeding risk) |
| Precautions |
|---|
| • Inferior vena cava and superior vena cava abnormalities• Presence of a bioprosthesis in the tricuspid or pulmonary valve• Presence of RV pacing leads• Right-sided congenital heart diseases• Prolonged cardiopulmonary resuscitation• Syncope (consider the possibility of head trauma) |
| Contraindications |
|---|
| • Absence of venous access• Presence of a mechanical prosthesis in the tricuspid or pulmonary valve• Head trauma• Active bleeding• History of cerebral hemorrhage or active brain tumor• Hemodynamic instability that does not permit safe treatment completion |
ESC, European Society of Cardiology; HR-PE, high-risk pulmonary embolism; IHR-PE, intermediate–high-risk pulmonary embolism; LT, local thrombolysis; RV, right ventricular; ST, systemic thrombolysis.
Evidence on local or intrapulmonary thrombolytic devices and different catheter-directed intervention techniques
| Study | Population | tPO | Intervention | Efficacy (PO) | Safety | NCT |
|---|---|---|---|---|---|---|
| Randomized studies | ||||||
| ULTIMA29 | IHR-PE,59 patients | 24 h | USAT vs heparin | RV/LV reduction0.30 vs 0.03 (P <.001) | 0 deaths0 MB | NCT01166997 |
| Kroupa et al.30 | IHR-PE23 patients | 48 h | LT vs heparin | RV/LV reduction0.88 vs 1.42 (P=.01) | 0 deaths0 MB | – |
| CANARY31 | IHR-PE94 patients* | 3 mo | LT vs heparin | RV/LV reduction0.7 vs 0.8 (P=.01) | 3 deaths (heparin)1 MB (LT) | NCT05172115 |
| SUNSET sPE34 | IHR-PE82 patients | 48 h | USAT vs conventional LT | Reduction in thrombi on CT (no differences, P=.76) | 1 death (USAT)2 MB (USAT) | NCT02758574 |
| HI-PEITHO33 | IHR-PE406 patients | 7 d | USAT vs heparin | MAEs | Currently enrolling patients | NCT04790370 |
| Observational studies | ||||||
|---|---|---|---|---|---|---|
| Seattle II35 | HR-PE (21%)IHR-PE (79%)150 patients | 48 h | USAT | RV/LV reduction 0.42±0.36 | 2.7% mortality at 30 d10% MB at 30 d | NCT01513759 |
| Spanish CDI Registry26 | HR-PE (37%)IHR-PE (63%)253 patients | At admission | LT 71%MT 53%Both 23% | Procedural success 91% | Mortality37.6% HR-PE2.5% IHR-PE | NCT06348459 |
| Portero et al.18 | HR-PE (11%)IHR-PE (89%)65 patients | 48 h | LT+aspiration+fragmentation(pharmacomechanical strategy) | Procedural success 90% | 2 deaths2 MB | – |
| KNOCOUT PE15 | IHR-PE489 patients | 72 h | USAT | – | 1% mortality at 30 d1.7% MB at 72 h | – |
| Meta-analysis | ||||||
|---|---|---|---|---|---|---|
| Zhang et al.36 | IHR-PE and HR-PE81 705 patients | – | Meta-analysis of heparin, ST, and LT | Lower mortality with LT than heparinOR=0.55 (95%CI, 0.39-0.80)Higher mortality with ST than LTOR=2.05 (95%CI, 1.46-2.89) | ICB same with LT and heparinOR=1.51 (95%CI, 0.75-3.04)Higher ICB with ST than LTOR=1.50 (95%CI, 1.13-1.99) | – |
CDI, catheter-directed intervention; CT, computed tomography; HR-PE, high-risk pulmonary embolism; ICB, intracranial bleeding; IHR-PE, intermediate–high-risk pulmonary embolism; LT, local thrombolysis; MB, major bleeding; MAEs, major adverse events; NCT, identifier at www.clinicaltrials.gov; RV/LV, ratio of the diameters of the right ventricle and left ventricle; tPO, time to assessment of primary objective (PO); USAT, local ultrasound-assisted thrombolysis.
This percutaneous procedure permits thrombus removal from the pulmonary circulation through aspiration and aims to restore flow in the pulmonary arteries and decrease the RV overload less invasively than surgical embolectomy (SE).
Dedicated devices. Two main MT systems are currently available. The FlowTriever system (Inari Medical, United States) comprises various catheters up to 24 Fr, a system for blood recovery, filtration, and return, and 2 self-expanding nitinol systems facilitating the organized extraction of thrombi. The system is advanced coaxially on an extra-support guidewire that should be positioned as distally as possible in the inferior lobes. In contrast, the 8-and 12-Fr Lightning Indigo and 16-Fr Lightning Flash systems (Penumbra, United States) are connected to a suction pump with a flow sensor that allows optimization of the aspiration efficacy, reducing blood loss. The system moves freely within the pulmonary artery anatomy. These 2 systems are described in figure 1. Table 5 summarizes the ideal candidate characteristics, precautions, and contraindications for this technique while table 6 reviews the evidence on MT.
Characteristics of patients with pulmonary embolism who are candidates for mechanical or catheter thrombectomy
| Good candidates | ||
|---|---|---|
| HR-PE | IHR-PE (rescue) | IHR-PE (elective) |
| • Contraindication for ST• Without contraindication for ST but with high bleeding risk with ST (BACS scale37)• ST or LT failure | • Clinical or hemodynamic deterioration according to ESC guidelines with anticoagulation as an alternative to ST due to its better safety profile | • In selected patients with IHR-PE, consider elective CDI if several poor prognostic factors (table 2) are present to prevent clinical or hemodynamic deterioration |
| Precautions |
|---|
| • Inferior vena cava and superior vena cava abnormalities• Presence of a bioprosthesis in the tricuspid or pulmonary valve• Previous thoracic radiation (higher perforation risk)• Presence of RV pacing leads• Right-sided congenital heart diseases• Chronic thromboembolic pulmonary hypertension (acute thrombus over chronic)• Presence of inferior vena cava filter• Thrombus-in-transit and patent foramen ovale• Avoid accessing distal branches with large-caliber devices• Monitor the amount of blood during extraction. Request cross-matching tests |
| Contraindications |
|---|
| • Absence of venous access• Presence of a mechanical prosthesis in the tricuspid or pulmonary valve• Disseminated pulmonary metastases• Active bleeding that contraindicates intraprocedural anticoagulation with sodium heparin• Peripheral thrombus |
ESC, European Society of Cardiology; HR-PE high-risk pulmonary embolism; IHR-PE, intermediate–high-risk pulmonary embolism; LT, local thrombolysis; RV, right ventricular; ST, systemic thrombolysis.
Evidence on percutaneous mechanical thrombectomy devices
| Study | Population | tPO | Description | Efficacy | Safety | NCT |
|---|---|---|---|---|---|---|
| FlowTriever | ||||||
| FLARE19 | IR-PE106 patients | 48 h | Single-arm studyFibrinolysis 2% | RV/LV reduction 0.38 | 3.8% MAEs1% MB0% ICB | NCT02692586 |
| FLAME38 | HR-PE115 patients | At admission | ObservationalFlowTriever vs medical therapy (ST 68.9%) vs performance goal | Composite MAEs17% vs 32% (P<.01)FlowTriever vs performance goal | Mortality1.9% with FlowTriever29.5% with medical therapy | NCT04795167 |
| FLASH16 | HR-PE (8%)IHR-PE (77%)800 patients | 48 h | ObservationalFibrinolysis 2.3% | mPAP reduction 7.6 mmHgRV/LV reduction 0.25 | 1.8% MAEs at 48 h0.3% mortality at 48 h | NCT03761173 |
| PEERLESS | IR-PE550 patients | 7 d | RandomizedFlowTriever vs LT | Composite MAEs at 7 d | Enrollment completed | NCT05111613 |
| PEERLESS 2 | IHR-PE1200 patients | 30 d | RandomizedFlowTriever vs heparin | Composite MAEs at 30 d | Currently enrolling patients | NCT06055920 |
| Penumbra Indigo | ||||||
|---|---|---|---|---|---|---|
| EXTRACT-PE20 | IR-PE119 patients | 48 h | Single-arm studyIndigo 8 Fr | RV/LV reduction 0.43 | 1.7% MAEs at 48 h0.8% mortality at 48 h | NCT03218566 |
| STORM-PE | IHR-PE100 patients | 48 h | RandomizedIndigo 12 Fr vs heparin | RV/LV reduction | Currently enrolling patients | NCT05684796 |
| STRIKE-PE | IHR-PE600 patients | 48 h | Single-arm studyLightning 12 and 16 Fr vs heparin | RV/LV reduction | Currently enrolling patients | NCT04798261 |
HR-PE, high-risk pulmonary embolism; ICH, intracranial hemorrhage; IHR-PE, intermediate–high-risk pulmonary embolism; IR-PE, intermediate-risk pulmonary embolism; LT, local thrombolysis; MAEs, major adverse events; MB, major bleeding; mPAP, mean pulmonary artery pressure; NCT, identifier at www.clinicaltrials.gov; RV/LV, ratio of the diameters of the right ventricle and left ventricle; tPO, time to assessment of the primary objective (PO).
Nondedicated devices and other peripheral thrombectomy systems used to treat PE are described in .
Technical details. The vascular access for all of these procedures is the femoral vein, unless it is thrombosed or the patient has a vena cava filter. Once access is obtained, anticoagulation should be maintained with UFH to achieve an activated clotting time of 250 to 300seconds.
There are no standardized criteria for MT completion. The objective of MT comprises clinical and hemodynamic parameters, meaning that a complete disappearance of the thrombus is not necessary to achieve a stable situation. Table 1 proposes objectives that can often be achieved with partial anatomical recanalization alone. Vascular closure can be performed with Perclose Prostyle or FemoStop (Abbott, United States), with Z- or figure of 8- suturing, or with the FlowStasis system included with the FlowTriever system.
Combination of techniques. Mechanical (fragmentation or MT) and pharmacological (LT) techniques can be used sequentially. There is little systematic evidence beyond registry data39 on the combined treatment and no randomized trials or comparisons between techniques. Some MT registries show anecdotal use of thrombolytics (2.3% in the FLASH trial).16 However, thrombolytics were used in 23.3% of cases in the corresponding Spanish registry.26 Theoretically, the combination of the techniques would be complementary, with MT immediately improving hemodynamic and respiratory parameters by eliminating large proximal thrombi and LT aiming to completely resolve the thrombus by acting on smaller branches. However, this strategy could increase the bleeding risk by combining large-caliber access with thrombolytic therapy. When this technique is used (particularly in MT as rescue from LT or ST), safe vascular access is vital (ultrasound-guided puncture, micropuncture).
Currently, combination treatment is not the strategy of choice in CDI but is reserved as rescue therapy. It is essential to select the appropriate technique for each individual patient from the outset to maximize benefits and minimize complications, taking into account the local experience and resources (figure 1 and figure 2).
Surgical embolectomySE outcomes depend on the patient's status and the experience of the center; in-hospital mortality ranges from 0% to 8% in patients with hypotension and from 22% to 44% in patients with cardiac arrest.40,41 There are no relevant randomized studies but guidelines still recommend SE as the first reperfusion alternative in patients with contraindications or fibrinolysis failure, with a class I C recommendation if resources are available and the teams are expert. Most tertiary centers lack optimal conditions for emergency SE 24hours a day, whereas they have CDI for acute PE,26,28 which is the true alternative to ST. SE can be considered a rescue treatment, both for patients with HR-PE and IHR-PE with hemodynamic deterioration and contraindication to or failure of other alternatives8,24,28 and possibly in the presence of a thrombus in transit in patients with patent foramen ovale ().
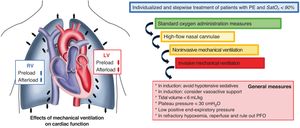
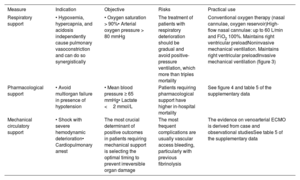
PERIPROCEDURAL SUPPORTRespiratory supportPulse oximetry should be used to continuously monitor oxygen saturation. An appropriate blood oxygen level should be maintained (arterial oxygen pressure >80mmHg and oxygen saturation >90%) using a nasal cannula, oxygen reservoir, high-flow nasal cannula, and noninvasive or invasive mechanical ventilation, in accordance with the recommendations in table 7 and figure 3.27,42,43
Respiratory support and periprocedural hemodynamics in intensive care units in patients with pulmonary embolism
| Measure | Indication | Objective | Risks | Practical use |
|---|---|---|---|---|
| Respiratory support | • Hypoxemia, hypercapnia, and acidosis independently cause pulmonary vasoconstriction and can do so synergistically | • Oxygen saturation > 90%• Arterial oxygen pressure > 80 mmHg | The treatment of patients with respiratory deterioration should be gradual and avoid positive-pressure ventilation, which more than triples mortality | Conventional oxygen therapy (nasal cannulae, oxygen reservoir)High-flow nasal cannulae: up to 60 L/min and FiO2 100%. Maintains right ventricular preloadNoninvasive mechanical ventilation. Maintains right ventricular preloadInvasive mechanical ventilation (figure 3) |
| Pharmacological support | • Avoid multiorgan failure in presence of hypotension | • Mean blood pressure ≥ 65 mmHg• Lactate <2 mmol/L | Patients requiring pharmacological support have higher in-hospital mortality | See figure 4 and |
| Mechanical circulatory support | • Shock with severe hemodynamic deterioration• Cardiopulmonary arrest | The most crucial determinant of positive outcomes in patients requiring mechanical support is selecting the optimal timing to prevent irreversible organ damage | The most frequent complications are usually vascular access bleeding, particularly with previous fibrinolysis | The evidence on venoarterial ECMO is derived from case and observational studiesSee |
ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen.
Management of hypoxemia in patients with pulmonary embolism admitted to the intensive care unit and effects of mechanical ventilation on cardiac function. Modified from Arrigo et al.42 LV, left ventricle; PE, pulmonary embolism; PFO, patent foramen ovale; RV, right ventricle; SatO2, oxygen saturation.
Vital signs (blood pressure, heart rate, and electrocardiogram) should be continuously monitored, as well as right heart catheterization parameters. After the procedure, monitoring is recommended for at least 24hours for IHR-PE and 48hours for HR-PE. An echocardiogram is recommended immediately after the procedure and at 24hours. If the patient is hypotensive, the measures included in table 7, , and figure 4 are recommended.8,42,44,45
Management of procedure-specific complicationsAlthough CDI is a safe procedure compared with ST, it is not free of complications.46 The most frequent are generally mild bleeding complications related to the vascular access and the anticoagulation. Although infrequent, iatrogenic perforation of the pulmonary arterial tree may range between mild and self-limiting hemoptysis and massive hemoptysis with hemodynamic and respiratory deterioration. The management of these patients necessitates reversal of the anticoagulation, balloon occlusion of the affected branch, and pulmonary angiography-mediated localization of the bleeding site to perform intravascular mechanical hemostasis (eg, prolonged balloon inflation, embolization). In life-threatening cases, orotracheal intubation is necessary with a dual-lumen tube to isolate the bleeding lung and permit complete ventilation of the contralateral lung.
Other rare but serious complications are RV perforation, tricuspid valve injury, and paradoxical embolism in patients with patent foramen ovale.
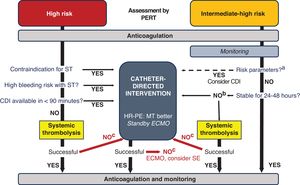
MANAGEMENT ALGORITHM FOR SEVERE PULMONARY EMBOLISMClinical practice guidelines recommend that the treatment of acute PE be based on the initial risk stratification,8 which should be conducted by a PERT. Local and regional protocols should be developed for decision-making. figure 5 shows the proposed comprehensive management algorithm, based on evidence and expert panel consensus, which reflects the current clinical practice in our centers. describes the approach in special situations (pregnancy, cardiac arrest, the postoperative period, cancer patients, and right heart thrombi).
Central illustration. Management algorithm for pulmonary embolism according to expert consensus. CDI, catheter-directed intervention; ECMO, extracorporeal membrane oxygenation; HR-PE, high-risk pulmonary embolism; MT, mechanical thrombectomy; PERT, pulmonary embolism response team; SE, surgical embolectomy; ST, systemic thrombolysis.
aParameters associated with normotensive shock or mortality ≥ 10% (table 2).
bAssess, as in HR-PE: contraindication for ST, high bleeding risk with ST, and CDI availability.
cEvaluate at 2 to 4h.
Early mortality in HR-PE exceeds 15% and is concentrated in the first hours of admission.47 Accordingly, emergency reperfusion therapy is recommended, with ST as the treatment of choice,8 despite limited evidence. MT is an increasingly popular technique due to its safety and efficacy (in the FLAME trial,38 MT was superior to the control medical therapy arm including ST, with in-hospital mortality of 1.9%). MT is currently indicated in patients with contraindications for fibrinolysis or after its failure,8 although the opinion of this expert panel is that it may also be useful in patients with high bleeding risk with ST, as assessed using the BACS scale.37 If MT is indicated in this context, it should be performed within a maximum of 60minutes from the decision if the patient is in a center with MT availability or within 90minutes if the patient requires transfer to a referral center (table 1).1 In addition, appropriate respiratory and hemodynamic support should be ensured at all times. LT can be recommended in selected patients if MT is unavailable (table 3).
Intermediate-high riskIn IHR-PE, anticoagulation without reperfusion therapy is sufficient for most patients, although normotensive patients with an elevated risk of hemodynamic deterioration require close monitoring8 because some may experience hemodynamic deterioration and require reperfusion (∼10% mortality with medical therapy). Current data indicate that CDI-related mortality in this setting is <3%,15–20,26 which is why this expert panel proposes the consideration of elective CDI in selected patients with IHR-PE with several poor prognostic factors to prevent this clinical deterioration. Patients with IHR-PE who are candidates for elective reperfusion are those meeting the criteria listed in table 2. Particularly relevant are the presence of syncope, elevated lactate, borderline blood pressure, and elevated heart rate.
In these patients, the first treatment should be anticoagulation, with consideration of elective reperfusion therapy during the first 24 to 48hours (table 1). The PERT should develop an individualized plan that includes the initial treatment, the alternatives, and the logistics necessary for reperfusion, including CDI.47
POSTDISCHARGE FOLLOW-UP OF ACUTE PEStudy of prothrombotic comorbiditiesThe distinction between transient and permanent comorbidities, and major and minor risk factors, influences the risk of recurrence and the need for chronic oral anticoagulation after a PE ().8,48
After a PE, anticoagulation should be maintained for at least 3 months. Subsequent treatment depends on the risk of recurrence, the risk of bleeding, and patient preferences. The established recommendations (independently of an eventual diagnosis of thrombophilia)48 are as follows:
- •
Withdraw the anticoagulation 3 months after a first PE event triggered by a major and resolved transient risk factor.
- •
Indefinite anticoagulation for a major permanent risk factor.
- •
Indefinite anticoagulation for men with idiopathic PE due to intermediate risk between the 2 previous groups.
- •
Additional studies () to determine whether anticoagulation should be maintained in women with idiopathic PE and for PE secondary to a minor and resolved transient risk factor, patients who wish to discontinue anticoagulation, and patients with an unknown risk/benefit relationship for indefinite anticoagulation.
Many patients have persistent dyspnea 3 months after anticoagulation for PE.49 Screening for CTEPD and chronic thromboembolic pulmonary hypertension (CTEPH) has prognostic and therapeutic value.
CTEPD is characterized by dyspnea on exertion, persistent perfusion deficits on ventilation/perfusion scintigraphy, normal resting pulmonary pressures, and exercise-induced pulmonary hypertension. Its true incidence is unknown. Screening should include cardiopulmonary exercise testing, in addition to echocardiography and lung scintigraphy. Confirmation requires resting and exercise catheterization.49,50
In addition, the diagnosis of CTEPH requires pulmonary arterial hypertension confirmed with resting right heart catheterization. Lifelong anticoagulation is necessary. Management comprises pulmonary thromboendarterectomy or pulmonary artery angioplasty, as well as specific pulmonary vasodilator therapy.50
Does reperfusion influence chronic thromboembolic pulmonary disease?The incidence of CTEPH after acute PE ranges between 2% and 5% in observational studies. However, the percentage of patients who develop CTEPD after acute PE is unknown. Although small studies indicated that ST can reduce the risk of CTEPH, a 3-year follow-up of 706 patients from the PEITHO trial revealed similar rates of CTEPH with ST and anticoagulation (2.1% vs 3.2%, P=.79).51 Studies with LT have found a greater short-term reduction in pulmonary artery systolic pressure,35,39 equaling that of the anticoagulation group during follow-up. No randomized studies have compared pulmonary pressure during follow-up in patients who remain symptomatic after acute PE treated with ST or CDI. Accordingly, there is insufficient evidence to state that reperfusion reduces the risk of CTEPH in patients with acute PE compared with anticoagulation.
CHARACTERISTICS OF THE CENTER AND EXPERT OPERATOR IN PULMONARY EMBOLISMCoordination among relevant professionals is required, using a PERT with protocols adapted to local resources to stratify risk and enable continuous, year-round, individualized care. The usefulness of cardiac surgery is debated; it has been proposed that referral centers with on-site cardiothoracic surgery should be established for urgent referrals from a PERT that lacks this resource. Proposals have been made to extend regional models such as the Infarction Code or Stroke programs to PE to enable effective and coordinated care (table 1).
Operator requirementsCDI in patients with PE is not free of risks; a crucial factor is interventional cardiologists’ ability to obtain safe vascular access, navigate to the right heart, and initiate ECMO. Other essential factors are operators’ understanding of acute patients and of the therapeutic options and specific devices. The initial concentration of experience in a small number of operators and the standardization of the procedure may improve outcomes but the experience should be disseminated within the team for occasional emergency situations.
CONCLUSIONSThis document summarizes the most relevant aspects of the management of HR-PE and IHR-PE from the perspective of cardiology and with a special focus on percutaneous techniques and periprocedural support. Where the clinical evidence is slight, the opinions of an expert panel are provided. The outcomes of patients with PE would be improved by a multidisciplinary diagnostic and therapeutic protocol, a systematic approach with risk stratification, early anticoagulation, and appropriate monitoring, as well as the implementation of new therapeutic strategies based on the evidence.
FUNDINGNone of the authors received funding for their contribution to the drafting of this article. The illustrations were made possible by an unconditional grant from Palex Medical.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used.
AUTHORS’ CONTRIBUTIONSP. Salinas: project administration, conceptualization, method, supervision, drafting of the original manuscript, manuscript revision. R. Martín-Asenjo: supervision, project administration, drafting of the original manuscript, manuscript revision. A.B. Cid Álvarez and J. Martín Moreiras: project administration, funding acquisition, drafting of the original manuscript, manuscript revision. P. Jorge Pérez and A. Viana-Tejedor: project administration, drafting of the original manuscript, manuscript revision. M.E. Vázquez-Álvarez, A. Jurado-Román, M. Juárez, M. Corbi-Pascual, M. Velázquez Martín, J. Jiménez-Mazuecos, S.O. Rosillo Rodríguez, V. Ruiz Quevedo, and M. Lázaro: drafting of the original manuscript and manuscript revision.
CONFLICTS OF INTERESTP. Salinas received honoraria for conferences and travel expenses for conferences from Inari Medical and Mercé Electromedicina and honoraria for conferences from Boston Scientific. The other authors do not declare conflicts of interest.
We thank Dr. Manuel Díaz for designing the illustrations and Dr. Antonia Delgado for providing the images in