Renal transplantation improves the survival and quality of life of patients with end-stage renal disease. Cardiovascular disease is the leading cause of morbidity and mortality in renal transplant recipients. The bidirectional relationship between renal and heart disease creates a unique clinical scenario that demands a comprehensive and personalized approach. This expert consensus, drafted by the Spanish Society of Transplantation, the Spanish Society of Cardiology, and the Spanish Society of Nephrology, aims to assess current practices and propose strategies for the management of heart disease in renal transplant recipients. A panel of Spanish nephrologists and cardiologists with expertise in renal and heart transplantation reviewed the scientific evidence concerning the current management of heart disease in renal transplant recipients. Subsequently, consensus statements were created through a 2-round Delphi methodology, resulting in 30 statements covering key topics such as the identification of renal transplant candidates, the management of heart disease in renal transplant recipients, and eligibility for combined heart-kidney transplantation in patients with both end-stage renal disease and cardiac disease. These consensus statements provide expert guidance for the management of heart disease in renal transplant recipients, an area where published clinical evidence remains limited.
Keywords
Renal transplantation (RT) has been demonstrated to improve the quality of life and survival in patients with end-stage renal disease (ESRD).1 However, cardiovascular disease is the leading cause of death after RT (35%-55% of the causes of death in RT recipients).2 Conventional risk factors (diabetes, hypertension, and dyslipidemia) and transplantation-specific risk factors (elevated levels of homocysteine, systemic inflammation, infections, and immunosuppressive drugs) drive the cardiorenal interaction and require a comprehensive and personalized approach.3
This bidirectional relationship between renal and heart disease requires collaboration between nephrologists and cardiologists for the management of patients with advanced heart disease and ESRD. RT recipients may experience changes in cardiovascular dynamics after renal function recovery,4 although they remain at elevated risk of cardiovascular events, such as coronary artery disease, heart failure, and arrhythmias.4 Therefore, more exhaustive and frequent cardiological and vascular evaluation before and after RT could help improve survival outcomes. The optimal management of this population is particularly challenging due to gaps in scientific evidence.
In this work, nephrologists and cardiologists who are experts in transplantation, in collaboration with the Spanish Society of Transplantation (SET), the Spanish Society of Cardiology (SEC), and the Spanish Society of Nephrology (SEN), explored the challenges associated with heart disease in RT recipients, examined current management strategies that can be used for consultation within Spain, and prepared consensus statements on renal and cardiac management in this setting.
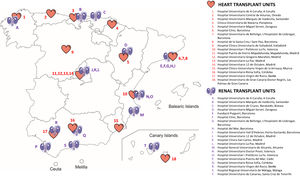
METHODSThis expert consensus involved nephrologists and cardiologists with expertise in transplantation, including participants from all heart transplantation (HT) units and an equivalent number of high-volume RT units in Spain (figure 1).
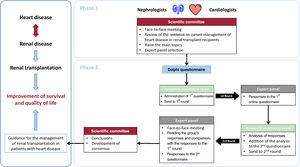
The consensus was developed in 2 phases (figure 2). Phase 1 consisted of a review of existing evidence on topics related to RT. Phase 2 involved a 2-round Delphi methodology (described elsewhere)5,6 to discuss the most controversial topics (or those with less supporting evidence) identified in Phase 1. The process was coordinated by 2 nephrologists (D. Hernández Marrero and J.M. Cruzado) and 2 cardiologists (M.D. García-Cosío and M. Farrero) and involved a scientific committee of 6 nephrologists and 6 cardiologists ().
Central illustration. Consensus development outline. Renal transplantation improves the survival and quality of life of patients with end-stage renal disease, but cardiovascular disease is the main cause of morbidity and mortality during renal transplantation. Given the bidirectional relationship between renal and heart disease, a comprehensive and personalized approach is needed. To guide the management of renal transplantation in patients with heart disease, an expert consensus among Spanish nephrologists and cardiologists with expertise in renal and heart transplantation was developed in 2 phases. Phase 1 involved a review of existing evidence on relevant topics related to renal transplantation, conducted by the scientific committee in a face-to-face meeting to raise the main topics. Phase 2 involved a 2-round Delphi methodology to discuss the most controversial topics (or those with less supporting evidence) identified in phase 1.
In phase 1, the scientific committee reviewed topics not addressed by current clinical practice guidelines,7,8 including: a) cardiac assessment of RT candidates; b) management of heart disease in RT; and c) HT candidacy in patients with ESRD. Evidence was presented in a face-to-face meeting in September 2023. Statements with full agreement from the scientific committee were approved, while those considered more controversial were submitted to the Delphi process. Evidence-supported statements were assigned a level of evidence and grade of recommendation according to the Scottish Intercollegiate Guidelines Network (SIGN) scale ().9
In October 2023, the first Delphi questionnaire (first round), comprising 30 statements, was sent to a panel of 15 experts in RT and 14 experts in HT (). These experts were selected based on their specialty (nephrologists and cardiologists) and their experience in the care of both RT and HT recipients (minimum of 5 years), as well as their scientific publications related to RT or HT.
Panelists provided their degree of agreement or disagreement with each statement using a 9-point Likert-type ordinal scale5 structured in 3 groups: 1-3, disagreement; 4-6, no agreement or disagreement; and 7-9, agreement. Median scores were obtained for each statement. Consensus of disagreement was inferred if the median score was 1-3 and ≥ 66.7% of respondents scored within this range; consensus of agreement was inferred if the median score was 7-9 and ≥ 66.7% of respondents scored within this range. Statements with a median score of 4-6 were considered uncertain by the majority of the group. In cases of disagreement or partial disagreement with the statement, panel members were asked to briefly explain their reasoning and were invited to rewrite the statement. Reformulated statements were discussed and a vote was held in a face-to-face meeting with the expert panel (November 2023) using the second Delphi questionnaire.
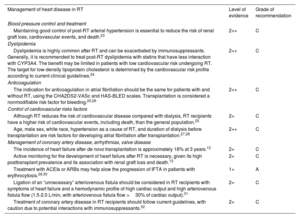
RESULTS AND DISCUSSION OF THE CONSENSUSCardiologic assessment of RT candidatesFive statements regarding the cardiologic assessment of candidates for RT were supported by clinical evidence (table 1) and 3 statements were submitted to the Delphi process (table 2), all of which reached consensus (82.2%-91.1%) in the first round.
Statements with committee agreement on the cardiological study of RT candidates
| Cardiological study of RT candidates | Level of evidence | Grade of recommendation |
|---|---|---|
| RT is the treatment of choice in patients with ESRD-associated heart disease.10,11 | 1+ | A |
| RT offers a significant survival advantage over other treatment options in patients with ESRD-associated heart disease.10,11 | 1+ | A |
| Patients on the waiting list for RT with advanced heart failure and a persistently low (< 30%) LVEF despite adequate fluid management on dialysis should be evaluated for combined (simultaneous or sequential) heart-kidney transplantation.8,12–14 | 2+ | C |
| RT candidates with heart failure and reduced ejection fraction should receive quadruple therapy with ACEIs/ARBs/sacubitril-valsartan, beta-blockers (preferably carvedilol), MRAs, and SGLT2i.12,15–17 | 2+ | C |
| Peritoneal dialysis or hemodialysis with shorter sessions or a more frequent weekly schedule should be prioritized in patients with ventricular dysfunction who are candidates for RT because they are more physiologic therapies.18 | 1+ | A |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ESRD, end-stage renal disease; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid-receptor antagonist; RT, renal transplantation; SGLT2i, sodium-glucose cotransporter type 2 inhibitor.
Controversial statements on the cardiological study of RT candidates submitted to Delphi consensus
| Cardiological study of RT candidates | % Agreement(Delphi round) |
|---|---|
| 1. All RT candidates should be evaluated on the basis of clinical history, physical examination, electrocardiogram, cardiac biomarkers, and echocardiogram. | 91.1%(1R) |
| 2. In the presence of proven chronic coronary artery disease, routine revascularization (percutaneous or surgical) to reduce perioperative risk is not warranted but should be assessed on a case-by-case basis. | 91.1%(1R) |
| 3. In patients with heart failure with reduced ejection fraction and no evident cardiologic cause, as well as advanced CKD on peritoneal dialysis or intermittent hemodialysis, an intensive hemodialysis program (with increased frequency and optimized parameters) should be considered to evaluate a potential uremic and reversible component of heart disease. | 82.2%(1R) |
1R, first round; CKD, chronic kidney disease; IQR, interquartile range; RT, renal transplantation.
According to the European Society of Cardiology (ESC)19 and Kidney Disease Improving Global Outcomes (KDIGO)8 guidelines, RT candidates without cardiologic symptoms should be assessed for cardiovascular disease through clinical evaluation, electrocardiogram, and chest X-ray. Those who have been or are on dialysis for at least 2 years, or have risk factors for pulmonary hypertension (eg, portal hypertension, connective tissue disease, chronic obstructive pulmonary disease, or congenital heart disease), should also undergo an echocardiogram. However, the timing for this assessment is not clearly established,20 and further research is needed to elucidate this issue.
There was agreement (91.1%) that all candidates for RT should be evaluated with an electrocardiogram alongside clinical history, physical examination, cardiac biomarkers (eg, N-terminal pro-B-type natriuretic peptide [NT-proBNP], troponin), and an echocardiogram (table 2). Biomarkers may be altered in chronic kidney disease (CKD) and patients on dialysis and should be used as a reference point for follow-up, especially considering the dynamic changes that can occur over time. Nevertheless, additional investigations are required.
Heart failure and reduced ejection fractionSolid data are available for quadruple therapy in RT candidates with heart failure and reduced ejection fraction (≤ 40%) with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers or sacubitril/valsartan, beta-blockers, mineralocorticoid-receptor antagonists (MRAs), and sodium-glucose cotransporter type 2 inhibitors (SGLT2i).12,15–17 Most drug classes are safe and effective in patients with heart failure with reduced ejection fraction and CKD up to stage 3b (estimated glomerular filtration rate [eGFR] minimum 30mL/min/1.73 m2).15 However, data are limited for those with stage 4-5 CKD as most of these patients are excluded from clinical trials.15 In patients with severe CKD (stage 4), there is some evidence of the safety and efficacy15 of SGLT2i, and to a lesser extent of angiotensin-converting enzyme inhibitors, vericiguat, digoxin, and omecamtiv mecarbil, but further clinical research is needed. In dialysis patients with dilated cardiomyopathy, carvedilol has been shown to increase 2-year survival and reduce morbidity and mortality.16 Sacubitril/valsartan has also been shown to improve left ventricular systolic and diastolic function in patients with heart failure with reduced ejection fraction and ESRD.17
Given the significant impact of left ventricular ejection fraction (LVEF) on RT candidacy, the expert panel developed a clinical protocol for up-titration of heart failure medication (figure 3). Adoption of this strategy may lead to better organ distribution due to a lower requirement for combined HT and RT (HRT) and may also increase RT candidacy in patients not eligible for HT if LVEF improvement is achieved.
Protocol for the management of heart failure in chronic kidney disease at different stages. ACEI, angiotensin-converting enzyme inhibitors; ARAII, angiotensin II receptor antagonists; ARNI, angiotensin receptor-neprilysin inhibitor; BP, blood pressure; GFR, glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; iSGLT2, sodium-glucose transport protein 2 inhibitor; MRA, mineralocorticoid-receptor antagonists.
In the last 2 decades, there has been a tendency to consider peritoneal dialysis therapy in ESRD patients with ventricular dysfunction and heart failure whenever possible, based on its potential to enhance quality of life for the patient, improve hemodynamic tolerance, and reduce hospital admissions.18 However, there are no randomized studies comparing peritoneal dialysis vs hemodialysis in ESRD patients with heart failure and reduced LVEF. According to the scientific committee, although home dialysis (peritoneal dialysis or home hemodialysis) may be considered as the first-line option,21 conventional hemodialysis may be required for better volumetric depletion or in cases of suboptimal dialysis efficacy with other techniques, as long as sudden changes in blood volume are avoided.
Although the existence of uremic heart disease has not been clearly characterized, there are reports of patients with eccentric left ventricular hypertrophy and significant systolic dysfunction that can be reversible with hemodialysis.18 In this regard, there was consensus among the panelists (82.2%) on the usefulness of intensive hemodialysis for patients with heart failure, reduced LVEF, and advanced CKD (table 2).
Coronary artery disease treatment in RT candidatesAfter the publication of the KDIGO 2020 guidelines,8 based on the results from the ISCHEMIA-CKD trial,22 in the subgroup of patients with eGFR <30mL/min/1.73 m2 or those on dialysis, an invasive strategy seems to be more favorable than a conservative strategy for severe baseline ischemia.22 There was a consensus (91.1%) among the panelists that revascularization should not be routine but decided on an individualized basis in patients with proven chronic coronary artery disease (table 2).
Management of heart disease in RT recipientsTen statements on the management of heart disease in RT were supported by clinical evidence (table 3), and 20 statements were submitted to the Delphi process. Of these, consensus was reached on 17 in the first round and on 3 after modification in the second round (table 4).
Statements with committee agreement on the management of heart disease in RT
| Management of heart disease in RT | Level of evidence | Grade of recommendation |
|---|---|---|
| Blood pressure control and treatment | ||
| Maintaining good control of post-RT arterial hypertension is essential to reduce the risk of renal graft loss, cardiovascular events, and death.23 | 2++ | C |
| Dyslipidemia | ||
| Dyslipidemia is highly common after RT and can be exacerbated by immunosuppressants. Generally, it is recommended to treat post-RT dyslipidemia with statins that have less interaction with CYP3A4. The benefit may be limited in patients with low cardiovascular risk undergoing RT. The target for low-density lipoprotein cholesterol is determined by the cardiovascular risk profile according to current clinical guidelines.24 | 2++ | C |
| Anticoagulation | ||
| The indication for anticoagulation in atrial fibrillation should be the same for patients with and without RT, using the CHA2DS2-VASc and HAS-BLED scales. Transplantation is considered a nonmodifiable risk factor for bleeding.25,26 | 2++ | C |
| Control of cardiovascular risks factors | ||
| Although RT reduces the risk of cardiovascular disease compared with dialysis, RT recipients have a higher risk of cardiovascular events, including death, than the general population.25 | 2+ | C |
| Age, male sex, white race, hypertension as a cause of RT, and duration of dialysis before transplantation are risk factors for developing atrial fibrillation after transplantation.27,28 | 2++ | C |
| Management of coronary artery disease, arrhythmias, valve disease | ||
| The incidence of heart failure after de novo transplantation is approximately 18% at 3 years.12 | 2+ | C |
| Active monitoring for the development of heart failure after RT is necessary, given its high posttransplant prevalence and its association with renal graft loss and death.12 | 2+ | C |
| Treatment with ACEIs or ARBs may help slow the progression of IFTA in patients with erythrocytosis.29,30 | 1+ | A |
| Ligation of an “unnecessary” arteriovenous fistula should be considered in RT recipients with symptoms of heart failure and a hemodynamic profile of high cardiac output and high arteriovenous fistula flow (1.5-2.0 L/min, with arteriovenous fistula flow >30% of cardiac output).31 | 2+ | C |
| Treatment of coronary artery disease in RT recipients should follow current guidelines, with caution due to potential interactions with immunosuppressants.32 | 2+ | C |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IFTA, interstitial fibrosis and tubular atrophy; RT, renal transplantation.
Controversial statements on the management of heart disease in RT submitted to Delphi consensus
| Management of heart disease in RT | % Agreement(Delphi round) |
|---|---|
| Blood pressure control and treatment | |
| 4. After RT (not peritransplantation), assessing blood pressure through ambulatory blood pressure monitoring and/or self-measurement of blood pressure is essential to rule out a nondipper pattern and/or masked hypertension. | 77.8%(1R) |
| 5. After RT, blood pressure should be measured at each visit. | 71.1%(1R) |
| 6. If systemic arterial pressure is ≥160/100 mmHg during the perioperative period of RT, hypotensive treatment should be initiated to reduce bleeding risk. | 86.7%(1R) |
| 7. Patients with chronic hypotension during the peritransplant period are at higher risk of primary failure and delayed graft function and may require treatment with vasoconstrictor drugs if necessary. | 75.6%(1R) |
| 8. In the long-term follow-up of patients undergoing RT, blood pressure control should aim for levels below 140/90 mmHg, or even 130/80 mmHg if the treatment is well tolerated. | 100%(1R) |
| 9. Treatment with ACEI or ARBs may help slow the progression of IFTA in patients with reduced tacrolimus levels (5-6 ng/mL). | 68.9%(1R) |
| 10. Thiazides are a useful option for calcineurin inhibitor-induced hypertension, but they may increase the risk of skin cancer due to their photosensitizing effect and should be reserved for third-line treatment (after renin-angiotensin system inhibitors and calcium antagonists). | 77.8%(1R) |
| 11. After RT, the decision to modify the type of immunosuppressive treatment to improve control of a specific cardiovascular risk factor must be weighed against the potential risk of rejection, worsening of other risk factors, and the limited data on the reduction in cardiovascular events. | 90.7%(2R) |
| 12. In cases of excessively high calcineurin inhibitor levels in hypertensive patients, the dose of the calcineurin inhibitor can be optimized before intensifying antihypertensive therapy. | 75.6%(1R) |
| 13. A management protocol should be established for patients receiving antiplatelet therapy who undergo RT due to the increased risk of peritransplant hemorrhage. | 93.3%(1R) |
| Dyslipidemia | |
| 14. PCSK9 inhibitors do not interfere with the metabolism of immunosuppressants and can be used in renal transplant patients at high cardiovascular risk who have not reached target lipid levels with statins/ezetimibe and/or who are intolerant to them. | 93.3%(1R) |
| 15. The type and dose of statins in both HT and RT should be selected based on the patient's renal function and potential drug interactions, especially with cyclosporine. It is recommended to start with lower doses and titrate until the target is reached, increasing to maximum doses if well tolerated. | 97.6%(2R) |
| 16. In patients at high cardiovascular risk with confirmed intolerance to high doses of statins who do not achieve the low-lipid lipoprotein target, combined therapies (statin at a lower dose plus ezetimibe and/or bempedoic acid and/or anti-PCSK9 monoclonal/siRNA antibodies) are recommended. | 97.6%(2R) |
| 17. Monitoring albuminuria after RT is important for classifying cardiovascular risk. | 93.3%(1R) |
| Anticoagulation | |
| 18. Direct-acting oral anticoagulants are not currently approved for dialysis, and their use in patients on the transplant waiting list is inadvisable due to potential difficulties with their reversal. | 75.6%(1R) |
| 19. Direct-acting oral anticoagulants are reasonable alternatives to vitamin K antagonists in adult renal transplant recipients, but evidence in solid organ transplant cohorts is limited. Those with the lowest renal clearance are preferred. | 86.7%(1R) |
| Management of coronary artery disease, arrhythmias, valve disease | |
| 20. Medical, surgical, or percutaneous treatment for acute and chronic coronary syndrome in renal transplant recipients follows the same indications as in the general population. | 97.8%(1R) |
| 21. The onset of heart failure within the first year after RT necessitates ruling out renal artery stenosis in the graft. | 88.9%(1R) |
| 22. Diagnosis of de novo heart failure in transplant recipients is the same as in the general population: clinical manifestations, natriuretic peptides, and evaluation of underlying heart disease. | 88.9%(1R) |
| 23. The treatment of de novo heart failure in renal transplant recipients is the same as in the general population, using medications that improve prognosis while assessing the potential reduction in glomerular filtration rate and associated hyperkalemia. | 100%(1R) |
1R, first round; 2R, second round; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IFTA, interstitial fibrosis and tubular atrophy; IQR, interquartile range; PCSK9, proprotein convertase subtilisin/kexin type 9; RT, renal transplantation; siRNA, small interfering RNA.
Several important factors related to blood pressure control in RT recipients were identified (table 4). Currently, no optimal blood pressure targets have been established in the peritransplant or follow-up periods, but chronic hypotension may hinder renal function recovery.33 This clinical issue, in which no pericarditis, pericardial effusion, amyloidosis or other causes of hypotension are detected, is not well understood pathophysiologically. The use of vasoactive drugs to maintain systolic pressure >100mm Hg (and close to 110 or 120mm Hg) may be beneficial in the immediate postoperative period.34 Conversely, high blood pressure may increase surgical RT bleeding and jeopardize long-term patient and graft survival.23 Randomized clinical trials are needed to clarify this concern.
Regarding optimal antihypertensive treatment, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers do not seem to have a prominent role (except in special situations), but there is considerable consensus on the use of calcium antagonists. There was no consensus to recommend any modification of immunosuppression after RT to improve systemic arterial pressure control or reduce cardiovascular events. Nevertheless, in RT recipients with high calcineurin inhibitor levels, dose optimization would be advisable before intensifying antihypertensive therapy (75.6% agreement).
Some studies in RT recipients have reported no change in the risk of cardiovascular events when switching from calcineurin inhibitors to mammalian target of rapamycin inhibitors.35,36 Considering this evidence, there was 90.7% agreement among panelists on the need to balance the potential cardiovascular benefits of modifying immunosuppression against the risks of rejection and the worsening of other cardiovascular risk factors.
DyslipidemiaThere was no consensus to modify the maximum statin dose in RT patients treated with calcineurin inhibitors (cyclosporine and tacrolimus) since the risk of rhabdomyolysis is low. It is important to consider the risk of drug-drug interactions when using statins and to select those that minimally interfere with cytochrome P450 3A4 (CYP3A4).37 While coadministering cyclosporine with specific statins may require a reduction in the statin dose, no adjustment is needed when combined with tacrolimus.38 Several studies have reported a significant decrease in the rate of cardiovascular events and mortality when statins are used in RT recipients.39,40 In HT recipients receiving tacrolimus, high-intensity statins are a safe option for the treatment of refractory hyperlipidemia.41 Thus, there was a 97.6% agreement among the panelists that the choice of statins in both HT and RT should consider patients’ renal function and potential drug-drug interactions.
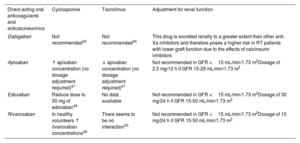
Atrial fibrillationFor patients with RT and atrial fibrillation, there is no evidence on the use of ablation and limited evidence on anticoagulants.42 Although evidence regarding direct-acting oral anticoagulants in RT is limited, the panelists agreed that these treatments are a reasonable alternative to vitamin K antagonists. Cyclosporine has been reported to increase rivaroxaban levels.43 However, a study reported no increase in bleeding events when combining cyclosporine with direct-acting oral anticoagulants in patients with atrial fibrillation.44 All oral anticoagulants have a varying percentage of renal elimination, so they may accumulate and increase the risk of bleeding if taken concomitantly with drugs that decrease their clearance. Therefore, their coadministration with immunosuppressants such as calcineurin inhibitors or mammalian target of rapamycin inhibitors (sirolimus and everolimus) requires careful monitoring.45 Specific dose adjustments of direct-acting oral anticoagulants are not required, although more frequent renal function monitoring should be performed (1-3 months after initiation and every 6-12 months thereafter, or more frequently based on patient-specific characteristics). Drugs that induce lower renal clearance should be avoided (eg, nonsteroidal anti-inflammatory drugs, high doses of diuretics, or immunosuppressants) if there is a degree of renal failure (GFR <50mL/min/1.73 m2).42,46Table 5 shows the possible combinations and interactions between direct-acting oral anticoagulants and anticalcineurinics.
Combination of direct-acting oral anticoagulants with anticalcineurinics
| Direct-acting oral anticoagulants and anticalcineurinics | Cyclosporine | Tacrolimus | Adjustment for renal function |
|---|---|---|---|
| Dabigatran | Not recommended45 | Not recommended45 | This drug is excreted renally to a greater extent than other anti-Xa inhibitors and therefore poses a higher risk in RT patients with lower graft function due to the effects of calcineurin inhibitors |
| Apixaban | ↑ apixaban concentration (no dosage adjustment required)47 | ↓ apixaban concentration (no dosage adjustment required)47 | Not recommended in GFR <15 mL/min/1.73 m2Dosage of 2.5 mg/12 h if GFR 15-29 mL/min/1.73 m2 |
| Edoxaban | Reduce dose to 30 mg of edoxaban48 | No data available | Not recommended in GFR <15 mL/min/1.73 m2Dosage of 30 mg/24 h if GFR 15-50 mL/min/1.73 m2 |
| Rivaroxaban | In healthy volunteers ↑ rivaroxaban concentrations49 | There seems to be no interaction50 | Not recommended in GFR <15 mL/min/1.73 m2Dosage of 15 mg/24 h if GFR 15-50 mL/min/1.73 m2 |
GFR, glomerular filtration rate; RT, renal transplantation.
There is a lack of evidence to rule out coronary artery disease in asymptomatic RT recipients.25 Risk factors associated with the occurrence of post-RT myocardial infarction are age, history of angina, peripheral vascular disease, dyslipidemia, pretransplant infarction, posttransplant hemoglobin decline, positive pretransplant noninvasive tests for ischemia, and arrhythmia.27,28 Persistently elevated troponin T levels, without normalization after restoration of renal function, were associated with an elevated risk of death and cardiovascular events at 5 years.51 However, there is no evidence to support the use of markers such as troponin T in the follow-up of RT patients.
There is no specific evidence regarding the treatment of coronary artery disease in RT recipients. The KDIGO guidelines suggest that management should be at least as intensive as in the general population52 and this was supported by the expert panel. Moreover, guidelines focus mainly on medical management and the use of statins and aspirin in cardiovascular disease.52 Primary prevention in diabetes with aspirin is suggested based on individual risk assessment and preferences. Additional well-designed studies are required to clarify this issue.
Heart failureNatriuretic peptides (brain natriuretic peptide [BNP], NT-proBNP) are important for screening de novo heart failure in RT recipients. Increases in plasma BNP after RT are associated with allograft dysfunction, while both pre- and posttransplant NT-proBNP levels have been linked to diastolic dysfunction and major cardiac adverse events.53,54 However, the predictive role of NT-proBNP in cardiac outcomes is uncertain due to multiple confounding factors (eg, degree of renal function).
There are few studies on the treatment of heart failure in RT recipients. In a small randomized clinical trial of RT recipients with left ventricular hypertrophy, lisinopril reduced left ventricular mass index compared with placebo.55 Randomized clinical studies are urgently needed to establish effective heart failure treatment following RT.
Despite this, all panelists agreed that the treatment of de novo heart failure in RT recipients should be the same as in the general population.56,57 However, the treatment may induce hyperkalemia in RT recipients, especially in those with renal tubular acidosis due to anticalcineurin drugs and with suboptimal graft function. In the absence of clear recommendations, therapies such as patiromer and sodium zirconium cyclosilicate require evaluation in RT recipients due to interference with drug absorption.12 Short reports have indicated that while zirconium cyclosilicate does not modify tacrolimus levels,58 patiromer may require an increase in tacrolimus dosage.59
Candidates for combined heart and renal transplantationTwo statements on the study of candidates for combined HRT were supported by clinical evidence (table 6) and 7 statements were submitted to the Delphi process; consensus was achieved for 4 statements in the first round and for 3 modified statements in the second round (table 7).
Statements with committee agreement on the study of candidates for RT and HT
| Study of candidates for combined RT and HT | Level of evidence | Grade of recommendation |
|---|---|---|
| Stable patients (elective and hemodynamically optimized) on dialysis, as well as those with GFR <30 mL/min/1.73 m2, benefit most from cardiorenal transplantation, with survival rates similar to those of patients with isolated HT. This strategy offers longer cardiac graft survival and a reduced need for dialysis/RT during follow-up.13,60 | 2+ | C |
| The prognosis of patients with severe heart disease and renal failure is poorer due to the lack of scientific evidence on optimal medical treatment and other devices as they are systematically excluded from studies.15 | 2+ | C |
GFR, glomerular filtration rate; HT, heart transplantation; RT, renal transplantation.
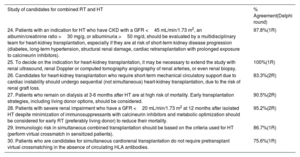
Controversial statements on the study of candidates for combined RT and HT submitted to Delphi consensus
| Study of candidates for combined RT and HT | % Agreement(Delphi round) |
|---|---|
| 24. Patients with an indication for HT who have CKD with a GFR <45 mL/min/1.73 m2, an albumin/creatinine ratio >30 mg/g, or albuminuria >50 mg/d, should be evaluated by a multidisciplinary team for heart-kidney transplantation, especially if they are at risk of short-term kidney disease progression (diabetes, long-term hypertension, structural renal damage, cardiac retransplantation with prolonged exposure to calcineurin inhibitors). | 97.8%(1R) |
| 25. To decide on the indication for heart-kidney transplantation, it may be necessary to extend the study with renal ultrasound, renal Doppler or computed tomography angiography of renal arteries, or even renal biopsy. | 100%(1R) |
| 26. Candidates for heart-kidney transplantation who require short-term mechanical circulatory support due to cardiac instability should undergo sequential (not simultaneous) heart-kidney transplantation, due to the risk of renal graft loss. | 83.3%(2R) |
| 27. Patients who remain on dialysis at 3-6 months after HT are at high risk of mortality. Early transplantation strategies, including living donor options, should be considered. | 90.5%(2R) |
| 28. Patients with severe renal impairment who have a GFR <20 mL/min/1.73 m2 at 12 months after isolated HT despite minimization of immunosuppressants with calcineurin inhibitors and metabolic optimization should be considered for early RT (preferably living donor) to reduce their mortality. | 95.2%(2R) |
| 29. Immunologic risk in simultaneous combined transplantation should be based on the criteria used for HT (perform virtual crossmatch in sensitized patients). | 86.7%(1R) |
| 30. Patients who are candidates for simultaneous cardiorenal transplantation do not require pretransplant virtual crossmatching in the absence of circulating HLA antibodies. | 75.6%(1R) |
1R, first round; 2R, second round; CKD, chronic kidney disease; GFR, glomerular filtration rate; HLA, human leukocyte antigen; HT, heart transplantation; RT, renal transplantation.
There is limited evidence on the prognosis of patients with severe heart disease and ESRD. A meta-analysis has reported an increase in cardiovascular mortality in patients with GFR <60-75mL/m/1.75 m2 and proteinuria.61 Heart failure is recognized as a major cause of morbidity and mortality in patients with CKD, with a 12 to 36 times higher risk of occurrence in those on dialysis compared with the general population; every 1-point increase in LVEF is associated with a 2.5% decrease in mortality among patients on the waiting list for RT.62
Key data on the current indication for HT in dialysis patients come from the United Network for Organ Sharing (UNOS)13 and the International Society for Heart and Lung Transplantation (ISHLT)63 registries. In the UNOS registry, HRT recipients had a lower adjusted risk of death compared with isolated HT recipients, especially among those on dialysis before transplantation.13 These data suggest that HRT should be considered in HT candidates with ESRD requiring dialysis and those with GFR <30mL/min/1.73 m2. Importantly, dialysis patients and those with GFR <30mL/min/1.73 m2 undergoing isolated HT were more unstable and had more ventricular assist devices than those undergoing combined transplantation.13
According to recommendations established in several consensuses, patients with a GFR <30mL/min/1.73 m2 are considered candidates for combined HRT, although some groups consider combined transplantation for patients with GFR <45mL/min/1.73 m2.14,60,64 At a consensus conference on HRT, Kobashigawa et al.14 evaluated indications for transplantation based on renal function. Combined transplantation is recommended for HT candidates with GFR <30mL/min/1.73 m2, while patients with GFR 30-45mL/min/1.73 m2 should be assessed individually. Patients with GFR >45mL/min/1.73 m2 should be considered for isolated HT. Similarly, Ahsan et al.60 have developed an algorithm for selecting patients who would benefit from combined HRT. Patients with eGFR <30mL/min/1.73 m2 with optimized hemodynamics criteria can be selected for combined HRT. Patients with eGFR 30 to 45mL/min/1.73 m2 are recommended combined HRT if they have acute kidney injury without complete recovery of renal function, that is, if they either remain on renal replacement therapy or have GFR <30mL/min/1.73 m2 after optimization. Patients with eGFR 30 to 45mL/min/1.73 m2 can also be considered for combined HRT if they have established CKD with small kidney size or proteinuria >0.5g/d.60 The American Heart Association provides similar criteria to Ahsan et al.60 for combined HRT.64
While the number of patients receiving combined HRT has increased in recent years, mortality has been reported to be up to 4.7-fold higher in HRT recipients than in those receiving RT after HT.65 Renal graft loss due to hemodynamic instability in the immediate posttransplantation period is a key issue with combined HRT. In the UNOS registry, the rate of primary graft failure at 5 years was 4% in HRT recipients and 2% in contralateral isolated kidney recipients.13 Furthermore, while the incidence of acute rejection is lower in combined transplantation, patients receiving RT after HT have the possibility of receiving a living donor transplant, offering advantages in terms of survival and increased health-related quality of life.65
Almost all panelists (97.8%) agreed that patients indicated for HT who have CKD and structural renal damage should be evaluated for HRT. However, while combined HRT improves prognosis in patients with advanced CKD and an indication for HT, the presence of functional cardiorenal injury (ie, reversible with cardiac improvement) is difficult to measure. Therefore, hemodynamic stabilization to enhance the evaluation of renal function is recommended whenever possible.
RT after isolated HTAccording to the expert panel, patients requiring short-term mechanical circulatory support due to cardiac instability should receive sequential HRT to mitigate the risk of graft loss. The panel acknowledged the high risk of mortality in HT patients with ESRD who are on long-term dialysis and the need to implement early RT strategies. The Canadian transplant registry indicates that HT recipients with ESRD have longer survival rates when they receive an RT compared with those who remain on the waiting list.66 In addition, UNOS registry data show that patients who received nonrenal transplants and later developed ESRD also have longer survival when they received an RT compared with those who remained on the waiting list. The risk of death or removal from the kidney transplant waiting list was also higher among candidates for RT after HT compared with those who received a RT alone.67 Importantly, it is difficult to predict the length of recovery from acute kidney injury in HT recipients and it may take several months.
However, prioritizing RT in HT recipients on dialysis may reduce opportunities for other patients on the waiting list. Currently, no studies have compared mortality in HT and non-HT recipients on dialysis.
HT recipients receive higher doses of calcineurin inhibitors in the first months after transplant, which can adversely affect renal function. Most panelists (95.2%) agreed that early RT, preferably with a living donor, should be considered in HT recipients with severe renal impairment, even with appropriate use of calcineurin inhibitors and metabolic optimization. However, waiting times for RT and access to living transplant programs vary by center. Thus, in cases of CKD with GFR <20mL/min/1.73 m2, which confer greater mortality risk in HT recipients, it is reasonable to consider early RT if feasible. An American consensus on HRT established a safety net for RT, prioritizing patients on dialysis after HT and those with persistent GFR ≤ 20mL/min/1.73 m2 for 6 weeks from day 30 to 365 after HT.14 Navarro-Manchón et al. reported that survival in patients with a GFR <30mL/min/1.73 m2 1 year after HT was significantly lower than in those with higher GFR.68 Notably, patients who received an RT had longer survival than those who remained on the waiting list,67 as has been demonstrated in patients with advanced CKD following HT.69
The immunologic risk in combined transplant should be based on HT criteria. A virtual crossmatch is required in sensitized patients but should not be performed in the absence of human leukocyte antigen (HLA) antibodies. According to the expert panel, all candidates for HRT should have their HLA antibodies tested periodically.
CONCLUSIONAfter 2 rounds of discussions and clarifications, all proposed statements were agreed upon with a high degree of consensus. However, some points needed clarification or refinement, many related to specific cases or differing perspectives among nephrologists and cardiologists. Nonetheless, the high level of agreement indicates that, despite the lack of evidence or the existence of controversies on some issues, health professionals managing patients undergoing RT clearly understand the importance of appropriate management, multidisciplinary collaboration, and further well-designed clinical studies to enhance patient care, which will impact survival and quality of life.
FUNDINGWe thank support from Instituto de Salud Carlos III (ISCIII), RICORS2040, RD21/0005/0001, RD21/0005/0010, RD21/0005/0012, RD21/0005/0022 “Financiado por la Unión Europea – NextGeneration EU”, Mecanismo para la Recuperación y la Resiliencia (MRR).
This study was funded by a nonconditioned grant from AstraZeneca, CSL Vifor, Astellas and Chiesi, who had no influence or participation in the development of this project, and by the Spanish Society of Transplantation, the Spanish Society of Cardiology, and the Spanish Society of Nephrology.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence has been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSM.D. García-Cosío, J.M. Cruzado, M. Farrero, and D. Hernández Marrero contributed equally to this work. M.D. García-Cosío, J.M. Cruzado, M. Farrero, and D. Hernández Marrero were responsible for the concept, writing—reviewing and editing, and final review of the work. M.T. Blasco Peiró, M. Crespo, J.F. Delgado Jiménez, B. Díaz Molina, C. Fernández Rivera, I.P. Garrido Bravo, V. López Jiménez, E. Melilli, S. Mirabet Pérez, M.L. Pérez Tamajón, D. Rangel Sousa, and E. Rodrigo Calabia performed the literature review, data collection, writing—reviewing and editing, and final review of the work. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTERESTM.D. García-Cosío has received speaker fees and travel grants from Astellas, AstraZeneca, Rovi, Abbott, Epycardio, and Chiesi. M. Farrero has received speaker fees and travel grants from Novartis, AstraZeneca, Rovi, Abbott, and Chiesi. S. Mirabet Pérez has received funding from Chiesi for participating in a lecture, consulting, and attending a congress. The remaining authors declare no conflicts of interest.
We are grateful to the cardiac and kidney transplant teams of the hospitals involved in this work and to AstraZeneca, CSL Vifor, Astellas and Chiesi, and thank the Spanish Society of Transplantation, the Spanish Society of Cardiology, and the Spanish Society of Nephrology for their support.
The authors would like to thank Ian Marshall on behalf of Springer Healthcare for providing medical writing assistance.