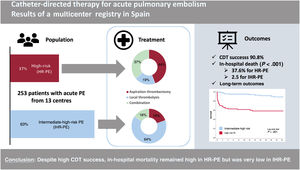
Catheter-directed therapy (CDT) for acute pulmonary embolism (PE) is an emerging therapy that combines heterogeneous techniques. The aim of the study was to provide a nationwide contemporary snapshot of clinical practice and CDT-related outcomes.
MethodsThis Investigator-initiated multicenter registry aimed to include consecutive patients with intermediate-high risk (IHR) or high-risk (HR), acute PE eligible for CDT. The primary outcome of the study was in-hospital all-cause death.
ResultsA total of 253 patients were included, of whom 93 (36.8%) had HR-PE, and 160 (63.2%) had IHR-PE with a mean age of 62.3±15.1 years. Local thrombolysis was performed in 70.8% and aspiration thrombectomy in 51.8%, with 23.3% of patients receiving both. However, aspiration thrombectomy was favored in the HR-PE cohort (80.6% vs 35%; P<.001). Only 51 patients (20.2%) underwent CDT with specific PE devices. The success rate for CDT was 90.9% (98.1% of IHR-PE patients vs 78.5% of HR-PE patients, P<.001). In-hospital mortality was 15.5%, and was highly concentrated in the HR-PE patients (37.6%) and significantly lower in IHR-PE patients (2.5%), P<.001. Long-term (24-month) mortality was 40.2% in HR-PE patients vs 8.2% in IHR-PE patients (P<.001).
ConclusionsDespite the high success rate for CDT, in-hospital mortality in HR-PE is still high (37.6%) compared with very low IHR-PE mortality (2.5%).
Keywords
Key aspects of the management of pulmonary embolism (PE) upon diagnosis include risk stratification, prompt use of anticoagulation and patient selection for reperfusion. Reperfusion can be defined as a treatment directed toward short-term reduction of thrombus burden and obstructive shock including: systemic thrombolysis, catheter-directed therapy (CDT), or surgical embolectomy. If systemic thrombolysis, which is the preferred method for reperfusion, is contraindicated or the risk of bleeding is considered prohibitive, CDT should be considered.1,2 In the European Society of Cardiology (ESC) 2019 guidelines for the diagnosis and management of acute pulmonary embolism,3 CDT is indicated in a) high-risk PE (HR-PE), when systemic thrombolysis is contraindicated or has failed (indication IIA, level of evidence C), and b) intermediate-high-risk PE (IHR-PE), when a patient on anticoagulation has hemodynamic deterioration as an alternative to systemic thrombolysis (indication IIA, level of evidence C). Given that fear of complications also contributes to the underuse of systemic thrombolysis, CDT could increase the number of patients undergoing reperfusion if clinically indicated.4
CDT can be categorized into 3 approaches: local thrombolysis (simple or ultrasound-assisted), aspiration thrombectomy (thrombus aspiration with or without fragmentation), and combined therapy (usually aspiration thrombectomy first followed by local thrombolysis).5,6 CDT as a reperfusion therapy was infrequently used until the 2010s due to limited indications in guidelines, lack of technical standardization, and disparities in therapy accessibility. However, the volume of procedures has increased significantly in the last 10 to 15 years both in Spain and globally.7–10 This increase can be attributed to 2 main factors: first, the increased awareness of undertreatment of patients requiring reperfusion1,11 and second, the emergence of new dedicated devices facilitating percutaneous intervention, with promising evidence in single-arm studies and small randomized clinical trials.12–14 Currently, CDT on submassive PE (equivalent to ESC intermediate-high and intermediate-low risk class) is frequently performed in the United States. On the other hand, the contemporary European scenario of PE management is widely heterogeneous, with fewer patients included in registries and a higher risk profile.15,16 Therefore, there is a need for systematic reporting of clinical data, ideally through multicenter registries that include consecutive patients. In this study, we report the first nationwide registry of CDT for the management of acute PE.
METHODSThe national registry of CDT for the management of acute PE is an investigator-initiated academic multicenter registry started in 2018 and is endorsed by the Spanish Interventional Cardiology Association (part of the Spanish Society of Cardiology). The registry included consecutive patients with IHR or HR acute PE (according to ESC guidelines risk stratification) who were eligible by local PE response teams (PERTs) for CDT. The registry design was prospective but centers with pre-existing local registries of PE patients treated with CDT that met the criteria for the national registry were allowed to include them retrospectively.
The inclusion criteria were as follows: a) adult patients (aged 18 years old or older), b) confirmed diagnosis of acute PE (on computed tomography or echocardiogram plus pulmonary angiography), c) high-risk PE or intermediate-high risk according to ESC risk stratification in the 2019 guidelines (briefly, elevated cardiac biomarkers [troponin or N-terminal pro-B-type natriuretic peptide], and right ventricle to left ventricle ratio>0.9 [on computed tomography or echocardiogram], and PE severity index III to IV or simplified PE severity index ≥ 1), and c) eligibility for CDT treatment. Exclusion criteria were a) unconfirmed diagnosis of PE, b) undefined risk stratification, and c) PE with uncertain chronology or beyond 7 days of symptoms initiation.
The primary outcome of the study was in-hospital all-cause death. Secondary outcomes were procedural success (defined as technical procedure completed without procedural complications or 48-hour death), in-hospital complications (defined as bleeding with International Society of Thrombosis and Hemostasis classification, repeat PE, post-CDT cardiac arrest, vascular complication, renal failure, or death), 1-month and 24-month all-cause death. Results were stratified by PE risk stratification. There is a knowledge gap on standardized definitions of outcomes for PE intervention studies, so there were no prespecified criteria for ending the CDT procedure, and the decision was taken by each CDT operator. The definition of systemic thrombolysis failure was also not standardized, but based on the limited previous literature and clinical judgement.5,17
Anonymized data were stored in a secure web-based database, and follow-up was performed as per local protocols at 1 month, 12 months, and 24 months. Data were self-reported by local investigators, and adverse events were audited for full disclosure. All patients provided informed consent. According to local research regulations, the registry protocol was accepted by the clinical research ethics committee in Hospital Clínico San Carlos (code 18/010-E), acting as the central ethics committee for all centers in Spain. The registry was purely observational, with no recommendation on PE management. The study was an academic, investigator-initiated study with no funding. Participating centers accepted an open invitation from the Spanish Interventional Cardiology Association that was not restricted to any subspecialty and included procedures performed by vascular surgery (1 center), interventional radiology (1 center) and interventional cardiology (11 centers) teams. This manuscript follows STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement for reporting of observational research.
There was no prespecified sample size for this study. The registry is ongoing, and in the present study, we present the first 253 patients included before September 30, 2022, from 13 Spanish centers. We used the Student t test and chi-square test (or the Fisher exact test when appropriate) to compare continuous and categorical variables, respectively. Kaplan-Meier curves were compared using the log-rank test. Statistical analyses were conducted with IBM SPSS Statistics version 22.
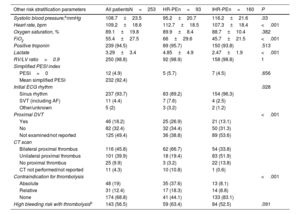
RESULTSBaseline characteristics and risk stratificationA total of 253 consecutive patients were included in the registry from 2014 to 2022, representing all CDT procedures performed at the 13 centers (an average of 5.86 procedures per year per million population, see table 1 of the supplementary data and figure 1 of the supplementary data). Ninety-three (36.8%) were HR-PE patients at the time of CDT, and 160 (63.2%) were IHR-PE patients. Baseline characteristics are shown in table 1. The mean age was 62.3±15.1 years, with a wide range between 16 and 86 years, and 45.5% were female. HR-PE patients were of similar age and had similar cardiovascular risk factors but more had frequently active cancer, recent surgery, or recent immobilization.
Baseline characteristics
| Baseline characteristics | All patientsN=253 | HR-PEn=93 | IHR-PEn=160 | P |
|---|---|---|---|---|
| Female sex | 115 (45.5) | 42 (45.2) | 73 (45.6) | .943 |
| Age | 62.3±15.1 | 61.79±16 | 62.6±14.7 | .692 |
| BMI | 29.2±5.9 | 28.04±5.94 | 29.86±5.76 | .025 |
| Obesity (BMI>30) | 81 (36.7) | 22 (26.5) | 59 (42.8) | .015 |
| Hypertension | 116 (45.8) | 37 (39.8) | 79 (49.4) | .140 |
| Diabetes | 47 (18.6) | 18 (19.3) | 29 (18.2) | .793 |
| Active smoker | 39 (15.5) | 16 (17.2) | 23 (14.4) | .634 |
| Chronic kidney disease | 23 (9.2) | 7 (7.6) | 16(10) | .672 |
| Prior stroke | 19 (7.6) | 10 (10.8) | 9 (5.6) | .056 |
| Contraceptive drugs | 17 (6.7) | 7 (7.5) | 10 (6.3) | .696 |
| Cancer diagnosis<5 y | 52 (20.7) | 26 (28) | 26 (16.3) | .013 |
| History of PE | 14 (5.5) | 3 (3.2) | 11 (6.9) | .221 |
| History of DVT | 27 (10.7) | 5 (5.4) | 22 (13.8) | .038 |
| Recent surgery | 46 (18.2) | 29 (31.2) | 17 (10.6) | <.001 |
| Recent immobilization | 54 (21.3) | 29 (31.2) | 25 (15.6) | .004 |
| COVID-19 | <.001 | |||
| <1 mo or active | 9 (3.6) | 3 (3.3) | 6 (3.7) | |
| 1 to 6 mo | 4 (1.6) | 1 (1.1) | 3 (1.9) | |
| Negative | 133 (52.6) | 66 (71) | 67 (41.9) | |
| NA (pre-COVID-19) | 107 (42.3) | 23 (24.7) | 84 (52.5) | |
| Primary symptom at PE admission | <.001 | |||
| Dyspnea | 128 (51) | 42 (45.2) | 87 (54.4) | |
| Syncope | 60 (23.9) | 22 (23.7) | 40 (25) | |
| Chest pain | 29 (11.6) | 4 (4.3) | 25 (15.6) | |
| Cardiac arrest | 16 (6.4) | 15 (16.1) | 0 | |
| Others | 9 (3.6) | 6 (6.5) | 3 (1.9) | |
| Combination of symptoms | 9 (3.6) | 5 (3.1) | 4 (4.3) |
BMI, body mass index; DVT, deep vein thrombosis; NA, not applicable; PE, pulmonary embolism.
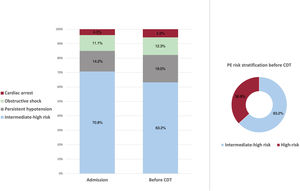
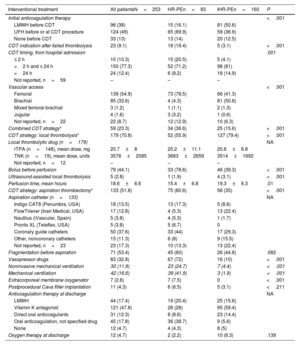
At admission, 74 (29.2%) patients were classified as HR-PE and 179 (70.8%) patients as IHR-PE. Of the 179 IHR-PE patients at admission, 19 (10.6%) had clinical deterioration meeting HR-PE criteria before undergoing CDT (figure 1A). For the purposes of this study, high-risk patients were considered those with HR-PE criteria as per the 2019 ESC guidelines before the CDT procedure: 93 (36.8%) patients (figure 1B), and 160 (63.2%) patients were classified as IHR-PE. Other risk stratification parameters are shown in table 2.
PE risk stratification parameters at admission
| Other risk stratification parameters | All patientsN=253 | HR-PEn=93 | IHR-PEn=160 | P |
|---|---|---|---|---|
| Systolic blood pressure,ammHg | 108.7±23.5 | 95.2±20.7 | 116.2±21.6 | .03 |
| Heart rate, bpm | 109.2±18.6 | 112.7±18.5 | 107.3±18.4 | <.001 |
| Oxygen saturation, % | 89.1±19.8 | 89.9±8.4 | 88.7±10.4 | .382 |
| FiO2 | 55.4±27.5 | 66±29.6 | 45.7±21.5 | <.001 |
| Positive troponin | 239 (94.5) | 89 (95.7) | 150 (93.8) | .513 |
| Lactate | 3.29±3.4 | 4.85±4.9 | 2.47±1.9 | <.001 |
| RV/LV ratio>0.9 | 250 (98.8) | 92 (98.9) | 158 (98.8) | 1 |
| Simplified PESI index | ||||
| PESI=0 | 12 (4.9) | 5 (5.7) | 7 (4.5) | .656 |
| Mean simplified PESI | 232 (92.4) | |||
| Initial ECG rhythm | .028 | |||
| Sinus rhythm | 237 (93.7) | 83 (89.2) | 154 (96.3) | |
| SVT (including AF) | 11 (4.4) | 7 (7.6) | 4 (2.5) | |
| Other/unknown | 5 (2) | 3 (3.2) | 2 (1.2) | |
| Proximal DVT | <.001 | |||
| Yes | 46 (18.2) | 25 (26.9) | 21 (13.1) | |
| No | 82 (32.4) | 32 (34.4) | 50 (31.3) | |
| Not examined/not reported | 125 (49.4) | 36 (38.8) | 89 (53.6) | |
| CT scan | ||||
| Bilateral proximal thrombus | 116 (45.8) | 62 (66.7) | 54 (33.8) | |
| Unilateral proximal thrombus | 101 (39.9) | 18 (19.4) | 83 (51.9) | |
| No proximal thrombus | 25 (9.9) | 3 (3.2) | 22 (13.8) | |
| CT not performed/not reported | 11 (4.3) | 10 (10.8) | 1 (0.6) | |
| Contraindication for thrombolysis | <.001 | |||
| Absolute | 48 (19) | 35 (37.6) | 13 (8.1) | |
| Relative | 31 (12.4) | 17 (18.3) | 14 (8.8) | |
| None | 174 (68.8) | 41 (44.1) | 133 (83.1) | |
| High bleeding risk with thrombolysisb | 143 (56.5) | 59 (63.4) | 84 (52.5) | .091 |
AF, atrial fibrillation; bpm, beats per minute; CT, computed tomography; DVT, deep vein thrombosis; ECG, electrocardiogram; FiO2, fraction of inspired oxygen; HR-PE, high-risk pulmonary embolism; IHR-PE, intermediate-high-risk pulmonary embolism; LV, left ventricle; mmHg, milimeters of mercury; PESI, pulmonary embolism severity index; RV, right ventricle; SVT; supraventricular tachycardia.
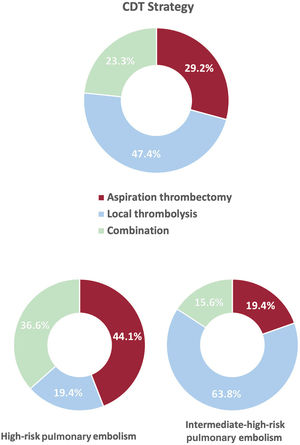
CDT was the elective reperfusion strategy in 90.9% of patients, and was a rescue therapy after failed thrombolysis in the remaining 9.1%. Anticoagulation was initiated before CDT in most patients, but unfractionated heparin was more frequently used in HR-PE than IHR-PE (table 3). Local thrombolysis was the most frequent method of CDT in the overall cohort (70.8%), followed by aspiration thrombectomy (51.8%), with 23.3% of patients receiving both therapies. However, aspiration thrombectomy was favored in HR-PE patients compared with IHR-PE patients (80.6% vs 35%, P<.001), as shown in table 3, figure 2, and figure 3. Most procedures (77.3%) were considered urgent (< 24hours from hospital admission), although 20.5% of HR-PE patients were treated<2hours from hospital admission. Median [interquartile range] procedural time was 60 [40-90minutes].
Interventional treatment
| Interventional treatment | All patientsN=253 | HR-PEn=93 | IHR-PEn=160 | P |
|---|---|---|---|---|
| Initial anticoagulation therapy | <.001 | |||
| LMWH before CDT | 96 (38) | 15 (16.1) | 81 (50.6) | |
| UFH before or at CDT procedure | 124 (49) | 65 (69.9) | 59 (36.9) | |
| None before CDT | 33 (13) | 13 (14) | 20 (12.5) | |
| CDT indication after failed thrombolysis | 23 (9.1) | 18 (19.4) | 5 (3.1) | <.001 |
| CDT timing, from hospital admission | .001 | |||
| ≤ 2 h | 10 (10.3) | 15 (20.5) | 5 (4.1) | |
| >2 h and ≤ 24 h | 150 (77.3) | 52 (71.2) | 98 (81) | |
| >24 h | 24 (12.4) | 6 (8.2) | 18 (14.9) | |
| Not reported, n=59 | – | – | – | |
| Vascular access | <.001 | |||
| Femoral | 139 (54.9) | 73 (78.5) | 66 (41.3) | |
| Brachial | 85 (33.6) | 4 (4.3) | 81 (50.6) | |
| Mixed femoral-brachial | 3 (1.2) | 1 (1.1) | 2 (1.3) | |
| Jugular | 4 (1.6) | 3 (3.2) | 1 (0.6) | |
| Not reported, n=22 | 22 (8.7) | 12 (12.9) | 10 (6.3) | |
| Combined CDT strategy* | 59 (23.3) | 34 (36.6) | 25 (15.6) | <.001 |
| CDT strategy: local thrombolysis* | 179 (70.8) | 52 (55.9) | 127 (79.4) | <.001 |
| Local thrombolytic drug (n=179) | NA | |||
| rTPA (n=148), mean dose, mg | 20.7±8 | 20.2±11.1 | 20.8±6.8 | |
| TNK (n=19), mean dose, units | 3576±2085 | 3663±2659 | 3514±1692 | |
| Not reported, n=12 | – | – | – | |
| Bolus before perfusion | 79 (44.1) | 33 (78.6) | 46 (39.3) | <.001 |
| Ultrasound-assisted local thrombolysis | 5 (2.8) | 1 (1.9) | 4 (3.1) | <.001 |
| Perfusion time, mean hours | 18.6±6.6 | 15.4±6.8 | 19.3±6.3 | .01 |
| CDT strategy: aspiration thrombectomy* | 133 (51.8) | 75 (80.6) | 56 (35) | <.001 |
| Aspiration catheter (n=133) | NA | |||
| Indigo CAT8 (Penumbra, USA) | 18 (13.5) | 13 (17.3) | 5 (8.6) | |
| FlowTriever (Inari Medical, USA) | 17 (12.8) | 4 (5.3) | 13 (22.4) | |
| Nautilus (iVascular, Spain) | 5 (3.8) | 4 (5.3) | 1 (1.7) | |
| Pronto XL (Teleflex, USA) | 5 (3.8) | 5 (6.7) | 0 | |
| Coronary guide catheters | 50 (37.6) | 33 (44) | 17 (29.3) | |
| Other, noncoronary catheters | 15 (11.3) | 6 (8) | 9 (15.5) | |
| Not reported, n=23 | 23 (17.3) | 10 (13.3) | 13 (22.4) | |
| Fragmentation before aspiration | 71 (53.4) | 45 (60) | 26 (44.8) | .082 |
| Vasopressor drugs | 83 (32.8) | 67 (72) | 16 (10) | <.001 |
| Noninvasive mechanical ventilation | 30 (11.9) | 23 (24.7) | 7 (4.4) | <.001 |
| Mechanical ventilation | 42 (16.6) | 39 (41.9) | 3 (1.9) | <.001 |
| Extracorporeal membrane oxygenator | 7 (2.8) | 7 (7.5) | 0 | <.001 |
| Postprocedural Cava filter implantation | 11 (4.3) | 6 (6.5) | 5 (3.1) | <.211 |
| Anticoagulation therapy at discharge | NA | |||
| LMWH | 44 (17.4) | 19 (20.4) | 25 (15.6) | |
| Vitamin K antagonist | 121 (47.8) | 26 (28) | 95 (59.4) | |
| Direct oral anticoagulants | 31 (12.3) | 8 (8.6) | 23 (14.4) | |
| Oral anticoagulation, not specified drug | 45 (17.8) | 36 (38.7) | 9 (5.6) | |
| None | 12 (4.7) | 4 (4.3) | 8 (5) | |
| Oxygen therapy at discharge | 12 (4.7) | 2 (2.2) | 10 (6.3) | .139 |
CDT, catheter-directed treatment; HR-PE, high-risk pulmonary embolism; IHR-PE, intermediate-high-risk pulmonary embolism; LMWH, low molecular weight heparin; NA, not applicable, descriptive purposes only; rTPA, alteplase. TNK, tenecteplase; UHF, unfractionated heparin.
Treatment strategy details are summarized in table 3. The access site was mostly femoral in HR-PE patients (78.5%), and nonfemoral (mainly antecubital veins) in IHR-PsE patients (58.7%). In our registry, only 51 patients (20.2%) underwent CDT with specific devices: 30.1% in HR-PE procedures and 14.4% in IHR-PE procedures (P<.003). The use of PE dedicated devices increased over time and reached 73% in 2022 (figure 1 of the supplementary data). The local thrombolysis strategy used mostly alteplase with a mean dose of 20.7±8mg over a mean of 18.6±6.6hours. Ultrasound-assisted thrombolysis was infrequent (2.8%) and used the EKOS system (Boston Scientific, USA). The aspiration thrombectomy strategy used specific PE devices in only 35.1% of patients, and most frequently (42%) used coronary guide catheters (6-Fr to 8-Fr). In the HR-PE subgroup, the need for cardiorespiratory support was frequent (72% vasopressors, 42% mechanical ventilation, 7.5% extracorporeal membrane oxygenator) but the need for support was rare in IHR-PE procedures.
Efficacy and safety of catheter-directed therapyThe success of CDT (defined as technical procedure completed without procedural complications or 48-hour death) was 90.9% (98.1% of IHR-PE patients vs 78.5% of HR-PE patients, P<.001). The investigators reported pre- and postinvasive measurements in 179 patients and pre- and postechocardiogram measurements in 67 patients (table 2 of the supplementary data). Hemodynamics improved significantly after the procedure, with a mean rise in systolic pressure of 10.5±20mmHg and a mean decrease in systolic pulmonary pressure of 11.8±11mmHg. Right ventricle diameter decreased by a mean of 6.1mm, and tricuspid annulus systolic excursion increased by 5.5mm (table 2 of the supplementary data). During CDT, there were 7 complications (2.8%), 4 of them in HR-PE patients (4.3% of CDT in HR-PE) and 3 in IHR-PE (1.9% of CDT in IHR-PE) but only 2 were definitely related to CDT device or procedure (table 3 of the supplementary data). There were 2 fatal complications, of which 1 was definitely related to CDT (cardiac tamponade due to direct injury from the catheter) and another was possibly related to CDT (atrioventricular block possibly traumatic but in the setting of profound obstructive shock).
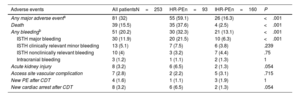
Clinical outcomesIn-hospital adverse events are shown in table 4 and were considerably higher in the HR-PE patients (59.1%) than in the IHR-PE patients (16.3%), P<.001. In-hospital mortality was 15.5% and was significantly higher in HR-PE patients (37.6%) than in IHR-PE patients (2.5%), P<.001. The causes of in-hospital death were PE-related shock/cardiac arrest in 21 patients (51.3%), fatal bleeding in 3 patients (7.7%), cancer in 3 patients (7.7%), other, non-PE-related causes in 10 patients (25.6%), cardiac tamponade plus PE-related shock in 1 patient (2.6%), and unknown in 1 patient (2.6%). The median [interquartile range] length of stay was 10 [8-16.5] days.
In-hospital adverse events
| Adverse events | All patientsN=253 | HR-PEn=93 | IHR-PEn=160 | P |
|---|---|---|---|---|
| Any major adverse eventa | 81 (32) | 55 (59.1) | 26 (16.3) | <.001 |
| Death | 39 (15.5) | 35 (37.6) | 4 (2.5) | <.001 |
| Any bleedingb | 51 (20.2) | 30 (32.3) | 21 (13.1) | <.001 |
| ISTH major bleeding | 30 (11.9) | 20 (21.5) | 10 (6.3) | <.001 |
| ISTH clinically relevant minor bleeding | 13 (5.1) | 7 (7.5) | 6 (3.8) | .239 |
| ISTH nonclinically relevant bleeding | 10 (4) | 3 (3.2) | 7 (4.4) | .75 |
| Intracranial bleeding | 3 (1.2) | 1 (1.1) | 2 (1.3) | 1 |
| Acute kidney injury | 8 (3.2) | 6 (6.5) | 2 (1.3) | .054 |
| Access site vascular complication | 7 (2.8) | 2 (2.2) | 5 (3.1) | .715 |
| New PE after CDT | 4 (1.6) | 1 (1.1) | 3 (1.9) | 1 |
| New cardiac arrest after CDT | 8 (3.2) | 6 (6.5) | 2 (1.3) | .054 |
CDT, catheter-directed therapy; HR-PE, high-risk pulmonary embolism; IHR-PE, intermediate-high risk pulmonary embolism; ISTH, International Society for Thrombosis and Haemostasis; PE, pulmonary embolism.
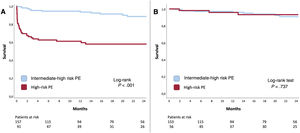
Follow-up data were available for 250 patients (98.8%) with a mean follow-up of 19.4±20 months. One-month survival was significantly higher in the IHR-PE group than in the HR-PE group: 1-month mortality was 1.3% vs 29.3% (P<.001). Long-term (24-month) mortality was lower at 8.2% vs 40.2% (P<.001), and Kaplan-Meier curves showed that most deaths occurred in the first month (figure 4A). Interestingly, estimation of long-term survival among patients alive at discharge showed that there were no differences in 24-month mortality between IHR-PE and HR-PE: 5.8% vs 5.3% (P<.001) (figure 4B), suggesting there was no late mortality burden after surviving a higher risk PE presentation.
DISCUSSIONThe study findings can be summarized as follows: a) Despite high CDT success, mortality was high in HR-PE patients (37.6% died during the index admission) but was very low in IHR-PE patients (2.5%). b) Approximately one-third of CDT for PE was performed in HR-PE patients compared with IHR-PE patients. The strategy varied significantly with 80% of HR-PE patients undergoing thrombus aspiration (with or without additional local thrombolysis) while 60% of IHR-PE patients received only local thrombolysis. c) Unlike stroke or myocardial infarction, a higher-risk presentation of the PE did not confer a worse long-term prognosis after the index admission.
High-risk pulmonary embolismContemporary studies show an in-hospital mortality rate of 6.5% in unselected PE patients (stable throughout 2016-2019 in a large United States administrative database).18 HR-PE in the same cohort, which includes several reperfusion strategies, including CDT, showed an in-hospital mortality decrease from 48.1% to 38.9%, similar to our HR-PE mortality (37.6%).18 Mortality in HR-PE patients is multifactorial and frequently derives from postcardiac arrest syndrome or multiorgan failure facilitated by obstructive shock.3 To improve these appalling figures, emergency services and in-hospital PERTs should coordinate to provide patients with prompt diagnosis and rapid access to reperfusion and supportive critical care.19,20 Evidence supporting CDT in this setting remains scarce, and our study is one of the largest series (n=93) of CDT in HR-PE. When dealing with unstable patients, time is a critical factor; consequently CDT timing and strategy should be discussed in detail. If CDT is to compete with systemic thrombolysis, which can virtually be administered seconds after decision, it must be provided promptly. In our study, only 20.5% of HR-PE patients underwent CDT in the first 2hours from admission (80%). A recent European consensus statement recommends that CDT should be initiated 60 to 90minutes after decision, or 2 to 4hours after completion of systemic thrombolysis (if considered failed); however, this was an expert consensus with little or no supporting evidence, and the issue should be clarified by further studies.5 In addition, time to benefit should be considered when choosing a CDT strategy. Local thrombolysis usually requires at least 12hours of infusion (although some faster protocols have been tested)21 but aspiration thrombectomy can provide hemodynamic improvement in<60minutes.13,14 Indeed, a preference for aspiration thrombectomy over local thrombolysis was present in our study (figure 3).
Intermediate-high-risk pulmonary embolismIHR-PE is an entirely different category that emerged in the 2014 ESC guidelines and attempted to encompass stable PE patients who merit special attention due to their risk of deterioration.22 Indeed, 7.2% of IHR-PE patients might die at 30 days,23 and 10.6% have an in-hospital adverse outcome (defined as PE-related death, cardiopulmonary resuscitation, or vasopressor treatment).24 Our study shows that CDT could help to reduce mortality: in-hospital mortality was 2.5% in the overall IHR cohort and was 0% in the PE dedicated devices subgroup. A randomized clinical trial with a medical treatment arm is warranted in this setting. However, in its absence, contemporary single-arm studies of CDT for IHR show very low mortality: 0.8% 30-day mortality in the FLASH registry,13 and 2.5% 30-day mortality in the Extract-PE study.14 Moreover, a meta-analysis comparing CDT vs systemic anticoagulation alone for submassive pulmonary embolism (n=9789) reported a significant decrease in in-hospital mortality, which was 2.4% with CDT vs 6.3% with medical treatment (relative risk, 0.41, 95% confidence interval (95%CI), 0.30-0.56, P<.00001)9; similarly, in a meta-analysis including 65 589 IHR and HR patients, CDT showed a 30-day mortality decrease compared with systemic thrombolysis 7.3 vs 13.6%; odds ratio, 0.51, 95%CI, 0.38–0.69, P<.001.10
Long-term outcomesFollow-up of our patients showed that long-term prognosis of HR-PE patients who survived to hospital discharge was no worse than that in patients with hemodynamic stability at admission (figure 4). This finding is markedly different to other major thrombotic syndromes such as myocardial infarction, in which infarct size correlates with 1-year all-cause mortality in patients discharged alive,25 myocardial infarction presenting with cardiogenic shock, which is also associated with higher 1-year and 5-year mortality in patients surviving beyond 30 days or hospital discharge,26 and stroke, in which stroke severity is independently associated with 1-year readmission.27 This finding supports the need to optimize the acute management of PE through dedicated in-hospital pathways (including, but not limited to, PERTs) and out-of-hospital local and regional networks such as stroke or myocardial infarction networks.
Catheter-directed therapy devicesAny new interventional technique requires time to develop successive iterations in the toolset and procedural planning. Access to pulmonary arteries requires safe crossing of the right heart and navigating through anatomical tortuosity. In this registry, 3 out of 253 patients (1.2%) had potential direct cardiac injury related to the catheters or devices themselves, 2 of them with fatal consequences (only one of those with a definite relationship). These adverse events should be adequately reported and closely monitored.
In addition, the size and frequent high level of organization of embolized thrombi do not facilitate extraction. Contemporary dedicated devices such as FlowTriever (Inari Medical, USA)28 or the Indigo System (Penumbra, USA)14,29 are specifically designed for this task, but were not available until 2022 (FlowTriever) and 2018 (Indigo 8-Fr). Our registry also shows a limited (but increasing) uptake of PE dedicated devices, likely related to incremental cost and the low number of cases per hospital, which limits the progression of the learning curve of these devices. Whether the overall increase in CDT procedures or the use of dedicated PE devices has impacted the outcomes of patients with PE remains unclear and warrants specific randomized clinical trials.
LimitationsThe registry has all the potential inherent biases of observational studies. However, we complied with STROBE standards, and the primary outcome was all-cause mortality, which is an outcome with a lower risk of bias. The potential selection bias might have been mitigated by including consecutive patients. However, we have no information on the total number of PE patients evaluated or admitted in the study period, and therefore a survival bias should be acknowledged because the registry represents only patients who survived to PERT evaluation and to the CDT procedure. To increase the number of patients included, the study period was long, which might have add heterogeneity to the treatment strategies. Although there was an open invitation for participation, the initiative originated from the Spanish Interventional Cardiology Association, and therefore interventional radiology is likely under-represented. The registry contained self-reported data and no external monitoring and so local investigators were responsible for the integrity of the data. Finally, the number of patients with dedicated PE devices was low (n=51). Therefore, this registry does not allow for interdevice comparisons.
CONCLUSIONSOne-third of CDT for PE was performed in HR-PE patients, using different strategies (80% of HR-PE patients underwent aspiration thrombectomy vs 60% of IHR-PE patients receiving local thrombolysis). Despite high CDT success, in-hospital mortality in HR-PE remained high (37.6%) compared with very low IHR-PE mortality (2.5%).
- -
Standard therapy for high-risk and selected patients with intermediate-high risk PE is reperfusion by means of systemic thrombolysis.
- -
However, formal contraindications and fear of complications contributes to underuse of systemic thrombolysis.
- -
CDT could increase the number of patients undergoing reperfusion if clinically indicated; however, there is wide heterogeneity in indications, access to therapy, strategies, devices, and results.
- -
CDT is feasible in HR-PE patients, although mortality remains high (37.6% died during index admission). Clinical practice favors thrombectomy in this setting.
- -
CDT for selected patients with intermediate-high risk PE is associated with excellent outcomes (2.5% mortality during index admission). The most frequent strategy was local thrombolysis.
- -
Unlike stroke and myocardial infarction, a higher-risk presentation of PE did not confer a worse long-term prognosis after the index admission.
None.
AUTHORS’ CONTRIBUTIONSP. Salinas and J. Jiménez-Mazuecos designed the study outline. P. Salinas designed the protocol and database, coordinated the data analysis and interpretation, and drafted the article. M.E. Vázquez-Álvarez, N. Salvatella, V. Ruiz Quevedo, M. Velázquez Martín, E. Valero, E. Rumiz, A. Jurado-Román, I. Lozano, F. Gallardo, I.J. Amat-Santos, Ó. Lorenzo, J.J. Portero Portaz, M. Huanca, L. Nombela-Franco, B. Vaquerizo, R. Ramallal Martínez, N.M. Maneiro Melón, J. Sanchis, A. Berenguer, A. Gallardo-López, E. Gutiérrez-Ibañes, H. Mejía-Rentería, and J. G. Córdoba-Soriano participated in data collection and also critically revised the article. All authors gave final approval of the version to be published.
CONFLICTS OF INTERESTJ. Sanchis is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial processing of the manuscript has been followed. The remaining authors declare no conflicts of interest regarding this manuscript.
The authors wish to thank the following members of the Pulmonary embolism response team of Hospital Clínico San Carlos, which contributes to continuous improvements in patient care and the development of this therapy: Noemí Ramos-López, Carlos Ferrera, Tania Sonia Luque Díaz, Daniel Enríquez-Vázquez, Patricia Mahía-Casado, Francisco Javier Noriega, Pablo Zulet Fraile, Fabián Islas, Pilar Jiménez-Quevedo, Nieves Gonzalo, Iván Nuñez-Gil, Gabriela Tirado, Fernando Macaya, Javier Escaned, Ana María Mañas Hernández, Laura Galván-Herráez, José María Pedrajas, José Bustamante, Jaime Abelaira, Mónica Pérez Serrano, Esther Bernardo García, María Aranzazu Ortega Pozzi, Javier Higueras, Alberto de Agustín, Ana Viana-Tejedor, Carlos Real, and Antonio Fernández-Ortiz.