Infective endocarditis (IE), first described by William Osler in 1885, is a serious disease.1 Classic forms of IE are mainly caused by Streptococcus viridans or Staphylococcus aureus.2 Numerous sociocultural and health-related changes (eg, population aging, complex cardiac surgery, a greater use of implanted pacemakers and defibrillators, and a higher prevalence of health care-associated bacteremia)3 have changed the face of IE in recent decades.4 As suggested in recent studies, one of the possible consequences of these changes is a shift in the microbiological profile of IE.4,5 The aim of this study was to analyze the causative microorganisms identified in native and prosthetic forms of IE at our hospital over a period of 33 years and to examine the changes that have taken place. We analyzed a cohort of patients with IE whose data were prospectively recorded between 1987 and 2019. The cohort included all patients diagnosed with IE during this period except persons who inject drugs. The study period was divided into 3 periods: 1987 to 1997, 1998 to 2008, and 2009 to 2019.
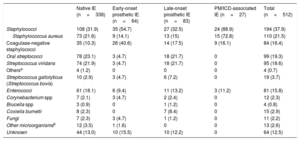
Our hospital is a tertiary care center with a cardiac surgery department that serves as an IE referral center for 3 regional hospitals in the province. Native IE accounted for 66.1% (n = 512) of all IE cases over the 33-year study period, prosthetic IE for 28.7% (12.5% early-onset and 16.1% late-onset), and pacemaker/implantable cardioverter defibrillator (PM/ICD)-associated IE for 5.2%. The mean±SD age of the patients was 55.3±17.9 years and 66.2% were men. There was a significant increase in IE cases detected in more recent years (138 in 1987-1997, 180 in 1998-2008, and 194 in 2009-2019; P<001). The causative microorganisms by period are shown in table 1. The most common microorganisms (accounting for 37.9% of all causative agents over the 33 years) were staphylococci (21.5% S aureus and 15.8% coagulase-negative staphylococci), streptococci (23%; 19.3% oral streptococci and 3.7% Streptococcusgallolyticus), enterococci (16.2%), and other (11.3%). The cause was unknown in 12.5% of cases. Staphylococci were the most common causative agents in all forms of IE, but they were particularly common in early-onset prosthetic IE—where they were responsible for 54.7% of all cases (40.6% coagulase-negative staphylococci)—and ICD/PM-associated IE, where they were responsible for 88.9% of all cases (72.8% S aureus) (table 1). Of the 110 cases of S aureus, 25 (22.7%) were methicillin-resistant. This high rate of S aureus infection is important, as recent studies have shown that S aureus is an independent predictor of poor outcome in IE.6 The microorganisms responsible for native IE and late-onset prosthetic IE were similar (table 1). Of note, a relatively high proportion of native IE and late-onset prosthetic IE cases were caused by Coxiella burnetii (2.3% and 8.4%, respectively).
Distribution of causative microorganisms in patients with IE for the period 1987 to 2019 (overall and by type)
| Native IE (n=338) | Early-onset prosthetic IE (n=64) | Late-onset prosthetic IE (n=83) | PM/ICD-associated IE (n=27) | Total (n=512) | |
|---|---|---|---|---|---|
| Staphylococci | 108 (31.9) | 35 (54.7) | 27 (32.5) | 24 (88.9) | 194 (37.9) |
| Staphylococcus aureus | 73 (21.6) | 9 (14.1) | 13 (15) | 15 (72.8) | 110 (21.5) |
| Coagulase-negative staphylococci | 35 (10.3) | 26 (40.6) | 14 (17.5) | 9 (16.1) | 84 (16.4) |
| Oral streptococci | 78 (23.1) | 3 (4.7) | 18 (21.7) | 0 | 99 (19.3) |
| Streptococcus viridans | 74 (21.9) | 3 (4.7) | 18 (21.7) | 0 | 95 (18.6) |
| Othersa | 4 (1.2) | 0 | 0 | 0 | 4 (0.7) |
| Streptococcus gallolyticus (Streptococcus bovis) | 10 (2.9) | 3 (4.7) | 6 (7.2) | 0 | 19 (3.7) |
| Enterococci | 61 (18.1) | 6 (9.4) | 11 (13.2) | 3 (11.2) | 81 (15.8) |
| Corynebacterium spp | 7 (2.1) | 3 (4.7) | 2 (2.4) | 0 | 12 (2.3) |
| Brucella spp | 3 (0.9) | 0 | 1 (1.2) | 0 | 4 (0.8) |
| Coxiella burnetii | 8 (2.3) | 0 | 7 (8.4) | 0 | 15 (2.9) |
| Fungi | 7 (2.3) | 3 (4.7) | 1 (1.2) | 0 | 11 (2.2) |
| Other microorganismsb | 12 (3.5) | 1 (1.6) | 0 | 0 | 13 (2.6) |
| Unknown | 44 (13.0) | 10 (15.5) | 10 (12.2) | 0 | 64 (12.5) |
ICD, implantable cardioverter defibrillator; IE, infective endocarditis; PM, pacemaker.
Values are expressed as No. (%).
Other microorganisms: Listeria monocytogenes (1 case), Lactobacillus spp. (1 case), Propionibacterium spp (1 case), enterobacteria (2 cases), HACEK microorganisms (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella (5 cases), and multiple pathogens (polymicrobial infection) (2 cases).
On analyzing the data by periods, we observed that the proportion of IE cases caused by staphylococci increased from 31.2% in 1987 to 1997 to 42.1% in 2009 to 2019; there was also a significant increase in coagulase-negative staphylococci (7.3% to 22.1%, P=.001) and a trend toward an increase in enterococci (10.8% to 19.5%, P=.089). There was no change in the proportion of cases caused by S aureus, oral streptococci, S gallolyticus, or unidentified microorganisms over the years (table 2). We did, however, observe a reduction (from 22.1% to 9.3%) of cases caused by rare microorganisms such as Brucella spp, C burnetii, Corynebacterium spp, and fungi (P<.001) (table 2). There was a significant increase in native IE cases due to coagulase-negative staphylococci (4.3% in 1987-1997 to 14.3% in 2009-2019) and a significant decrease in those due to S aureus (29.8% to 19.8%) and rare microorganisms (17.0% to 10.2%). The proportion of other causative microorganisms did not change (table 2). In the case of early-onset prosthetic IE, we observed a notable increase in cases due to coagulase-negative staphylococci (15% to 52.2%) and a decrease in those due to Corynebacterium spp (15% to 0%) and unidentified microorganisms (20% to 8.7%). The proportion of cases caused by other microorganisms did not change (table 2). Finally, in the case of late-onset prosthetic IE, there was an increase in cases caused by staphylococci (S aureus and coagulase-negative staphylococci) and enterococci (from 0% to 21.9%) and a notable decrease (36.4% to 12.5%) in those caused by oral streptococci (table 2).
Distribution of causative microorganisms (overall and by type) in patients with infective endocarditis for 1987 to 1997, 1998 to 2008, and 2009 to 2019
| All cases of infective endocarditis | 1987-1997 (n=138) | 1998-2008 (n=180) | 2009-2019 (n=194) | Pa |
|---|---|---|---|---|
| Staphylococci | 43 (31.2) | 71 (39.4) | 80 (42.1) | .152 |
| Staphylococcus aureus | 33 (23.9) | 39 (21.7) | 38 (20.0) | .637 |
| Coagulase-negative staphylococci | 10 (7.3) | 32 (17.7) | 42 (22.1) | .001d |
| Oral streptococci | 29 (16.7) | 37 (20.5) | 33 (17.0) | .579 |
| Streptococcus viridans | 29 (16.7) | 35 (19.4) | 31 (15.9) | .642 |
| Otherb | 0 | 2 (1.1) | 2 (1.1) | .816 |
| Streptococcus gallolyticus (Streptococcus bovis) | 6 (4.3) | 6 (3.3) | 7 (3.6) | .748 |
| Enterococci | 15 (10.8) | 29 (16.1) | 37 (19.5) | .089 |
| Other microorganismsc | 29 (22.1) | 10 (5.4) | 16 (9.3) | <.001d |
| Not identified | 16 (11.6) | 27 (15.0) | 21 (11.1) | .486 |
| Native infective endocarditis | 1987-1997 (n=94) | 1998-2008 (n=118) | 2009-2019 (n=126) | Pa |
|---|---|---|---|---|
| Staphylococci | 32 (34.1) | 33 (27.9) | 43 (34.1) | .364 |
| S aureus | 28 (29.8) | 20 (16.9) | 25 (19.8) | .048d |
| Coagulase-negative staphylococci | 4 (4.3) | 13 (11.0) | 18 (14.3) | .049d |
| Oral streptococci | 20 (21.3) | 31 (26.3) | 27 (21.4) | .486 |
| S viridans | 20 (21.3) | 29 (24.6) | 25 (19.8) | .524 |
| Other | 0 | 2 (1.7) | 2 (1.6) | .841 |
| S gallolyticus (S bovis) | 3 (3.2) | 2 (1.7) | 5 (3.9) | .712 |
| Enterococci | 12 (12.7) | 25 (21.2) | 24 (19.0) | .267 |
| Other microorganisms | 16 (17.0) | 8 (6.7) | 13 (10.2) | .047d |
| Not identified | 11 (12.2) | 19 (16.1) | 14 (11.1) | .484 |
| Early-onset prosthetic infective endocarditis | 1987-1997 (n=20) | 1998-2008 (n=21) | 2009-2019 (n=23) | Pa |
|---|---|---|---|---|
| Staphylococci | 5 (25.0) | 15 (68.2) | 15 (65.2) | .005d |
| S aureus | 2 (10.0) | 4 (19.0) | 3 (13.0) | .474 |
| Coagulase-negative staphylococci | 3 (15.0) | 11 (49.2) | 12 (52.2) | .019d |
| Oral streptococci | 1 (5.0) | 0 | 2 (8.7) | .746 |
| S viridans | 1 (5.0) | 0 | 2 (8.7) | .746 |
| Other | 0 | 0 | 0 | - |
| S gallolyticus (S bovis) | 2 (10.0) | 1 (4.7) | 0 | .641 |
| Enterococci | 3 (15.0) | 0 | 3 (13.0) | .676 |
| Other microorganisms | 5 (20.0) | 1 (4.7) | 1 (4.3) | .095 |
| Unknown | 4 (20.0) | 4 (19.0) | 2 (8.7) | .520 |
| Late-onset prosthetic infective endocarditis | 1987-1997 (n=22) | 1998-2008 (n=29) | 2009-2019 (n=32) | Pa |
|---|---|---|---|---|
| Staphylococci | 4 (18.2) | 11 (37.9) | 12 (37.5) | .083 |
| S aureus | 1 (4.5) | 8 (27.6) | 4 (12.5) | .088 |
| Coagulase-negative staphylococci | 3 (13.7) | 3 (10.3) | 8 (25.0) | .436 |
| Oral streptococci | 8 (36.4) | 6 (20.7) | 4 (12.5) | .110 |
| S viridans | 8 (36.4) | 6 (20.7) | 4 (12.5) | .110 |
| Other | 0 | 0 | 0 | — |
| S gallolyticus (S bovis) | 1 (4.5) | 3 (10.3) | 2 (6.2) | .369 |
| Enterococci | 0 | 4 (13.8) | 7 (21.9) | .048d |
| Other microorganisms | 8 (34.1) | 1 (4.7) | 2 (6.2) | <.001d |
| Unknown | 1 (4.5) | 4 (13.8) | 5 (15.6) | .485 |
Values are expressed as No. (%).
Statistical comparisons were made using the chi-square test or, for categories with frequencies <5, the Fisher-Freeman-Halton test.
Other microorganisms: Corynebacterium spp (12 cases), Coxiella burnetii (15 cases), Brucella spp (4 cases), fungi (11 cases), Listeria monocytogenes (1 case), Lactobacillus spp (1 case), Propionibacterium spp (1 case), enterobacteria (2 cases); HACEK microorganisms (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella (5 cases), and multiple pathogens (polymicrobial infection) (2 cases).
Our findings from a large series of IE cases spanning 33 years shows how the microbiological profile at our hospital has changed very significantly over time, with a notable increase in cases caused by coagulase-negative staphylococci and enterococci, practically no change in cases caused by oral streptococci, and a reduction in cases caused by rare microorganisms, such as Brucella spp, C burnetii, and Corynebacterium spp, which were all relatively common in the last century. Despite some slight differences, the changes in microbiological profile affected all types of IE. Our findings may have prognostic implications and lead to changes in the choice of empirical antibiotic therapy.