Postcardiotomy cardiogenic shock (PCCS) continues to be linked to high morbidity and mortality.1 Despite advances in the development of biotechnological resources, mortality figures have not shown a clear improvement during the last decade.1 Furthermore, survival rates of PCCS continue to be significantly lower than those observed in other types of cardiogenic shock (CS).2 This could potentially change with the implementation of dedicated structures specifically designed for CS treatment.3
We performed an observational analysis of a series of adult patients with PCCS treated after the establishment of an organized interdisciplinary shock-team. All consecutive patients were prospectively included, whether from our own center or referred to from other hospitals. Clinical follow-up covered a time period from September 2014 through to June 2019.
Bivariate analysis was performed of factors associated with in-hospital mortality. The Mann-Whitney test was used for numeric variables, and the chi-square test for categorical variables. Actuarial survival analysis used Kaplan-Meier curves and the log-rank test for comparison. The baseline shock variables used were those taken on admission in our intensive care unit (ICU). A value of P<.05 was considered statistically significant. The program used for the analysis was STATA IC/15.
The most representative results are displayed in table 1. A total of 32 PCCS patients were analyzed. Twenty-six cases (81%) occurred in our hospital, while 6 (19%) were referred from other institutions. In 31 patients (97%), a temporary mechanical circulatory support (TMCS) was used. Extracorporeal membrane oxygenation (ECMO) was chosen in 24 (75%), with central cannulation in 20 patients (83% of ECMOs used). The TMCS was implanted during the surgery itself in 68% of the patients, and on the same day in 87%. The median [range] time on circulatory mechanical support was 6 [5-14] days.
Demographic features, clinical management, complications, outcome and destination in patients with postcardiotomy cardiogenic shock
| Variable | Global PCCSn=32 | Group APCCSa survivorsn=20 | Group B PCCSa nonsurvivorsn=12 | P |
|---|---|---|---|---|
| Demographic | ||||
| Age, y | 59± 17 | 55±18 | 65±12 | .118 |
| Male sex | 21 (66) | 13 (65) | 8 (67) | .923 |
| BMI, kg/m2 | 28±8 | 28±9 | 29±4 | .103 |
| Clinical history | ||||
| Hypertension | 17 (53) | 10 (50) | 7 (58) | .647 |
| Diabetes | 7 (22) | 1 (5) | 6 (50) | .003 |
| History of stroke | 6 (19) | 1 (5) | 5 (42) | .010 |
| Cardiac surgery in our center | 26 (81) | 15 (75) | 11 (92) | .242 |
| Emergency surgery | 6 (19) | 4 (20) | 2 (17) | .815 |
| Type of surgery | .088 | |||
| CABG | 2 (6) | 0 (0) | 2 (17) | |
| Valve surgery | 16 (50) | 9 (45) | 7 (58) | |
| CABG+valve surgery | 12 (38) | 10 (50) | 2 (17) | |
| CABG+others | 1 (3) | 1 (5) | 0 (0) | |
| Others | 1 (3) | 0 (0) | 1 (8) | |
| Clinical variables | ||||
| ECC time, min | 190 (165-268) | 197 (173-272) | 175 (157-268) | .408 |
| MAPb, mmHg | 70 (58-82) | 71 (58-82) | 68 (58-81) | .533 |
| HR,b bpm | 95 (85-105) | 93 (82-100) | 98 (90-103) | .266 |
| PaO2/FiO2b | 247 (165-323) | 266 (229-321) | 198 (165-323) | .311 |
| VISc 24 hd | 34 (12-53) | 32.2 (7.7-52.5) | 33.9 (25-90) | .454 |
| VISc 48 hf | 12 (4-43) | 9.6 (2.4-33.8) | 30 (9-55) | .220 |
| SOFA 24 hd | 11 (10-12) | 10 (9-11) | 11 (11-12) | .08 |
| SAPS II 24 hd | 41 (32-45) | 36 (30-41) | 46 (41-54) | .003 |
| APACHE II 24 hd | 18 (15-24) | 18 (14-22) | 20 (17-25) | .182 |
| Laboratory results | ||||
| Lactate,b mmol/L | 11 (5-16) | 7 (4-14) | 14 (11-20) | .010 |
| Lactate 24 h,d mmol/L | 2.5 (1.4-4.5) | 1.7 (1.15-2.9) | 5.2 (2.7-9.8) | .022 |
| Peake lactate, mmol/L | 11.3 (5.3-17.6) | 5.6 (3.9-14.4) | 16.1 (12.1-20) | .005 |
| Creatinine,b mg/dL | 1.3 (0.8-1.5) | 1.3 (0.9-1.5) | 1.6 (1-1) | .267 |
| Creatinine 24 h,d mmol/L | 1.4 (1.1-2.13) | 1.2 (0.8-1.7) | 2.1 (1.1-2.1) | .024 |
| Peak creatinine,e mg/dL | 1.6 (1.3-2.5) | 1.5 (1-1.8) | 2.5 (1.8-3) | .005 |
| Blood glucose,b mg/dL | 232 (172-274) | 199 (159-255) | 260 (232-294) | .047 |
| Total bilirrubin, mg/dL | 1 (0.7-1.6) | 1 (0.7-1.9) | 1 (0.8-1.4) | .799 |
| Peake bilirrubin, mg/dL | 1.9 (1.4-3.2) | 2 (1.5-4) | 1.7 (1.3-3.2) | .838 |
| ALT,b mg/dL | 37 (20-318) | 34 (19-190) | 147 (20-1957) | .302 |
| AST,b mg/dL | 104 (65-325) | 104 (65-331) | 104 (65-240) | .901 |
| PaO2/FiO2b | 247 (165-323) | 266 (229-321) | 198 (165-323) | .311 |
| WBC,b×109/L | 13.7 (9.4-18.4) | 13.9 (10-17) | 12 (9-20) | .800 |
| Hemoglobinb, g/dL | 9.2 (8.4-10.4) | 9.1 (8.4-10.5) | 9.5 (8.4-10.1) | .922 |
| Peak procalcitonine,e ng/mL | 10.1 (1.3-24.2) | 5.8 (1-15.7) | 29.3 (9.7-69.1) | .019 |
| Clinical managment | ||||
| Use of IABP | 24 (75) | 16 (80) | 8 (67) | .399 |
| Use of TMCS | 31 (97) | 19 (95) | 12 (100) | .431 |
| Implant during surgeryg | 21 (68) | 12(63) | 9 (75) | .492 |
| TMCSgdevice | .621 | |||
| VA ECMO | 24 (75) | 15 (75) | 9 (75) | |
| Central VA ECMO | 20 (63) | 13 (65) | 7 (58) | |
| Peripheral VA ECMO | 4 (13) | 2 (10) | 2 (17) | |
| Centrimag Levitronix | 6 (19) | 4 (20) | 2 (17) | |
| Impella CP | 1 (3) | 0 (0) | 1 (8) | |
| Support time, dg | 6 (5-14) | 9 (5-14) | 6 (2-14) | .501 |
| TMCSgcomplications | ||||
| > 1 complicationsh | 23 (74) | 14 (70) | 9 (75) | .935 |
| Neurological events | .048 | |||
| Ischemic stroke | 2 (6) | 0 | 2 (17) | |
| Hemorrhagic stroke | 1 (3) | 0 | 1 (8) | |
| Encephalopathy | 4 (13) | 1 (5) | 3 (25) | |
| Others | 1 (3) | 1 (5) | 0 | |
| Tracheostomy | 10 (31) | 8 (40) | 2 (17) | .134 |
| Use of RRT | 14 (44) | 6 (30) | 8 (67) | .043 |
| ICU admission time, d | 18 (11-31) | 27 (15-40) | 10 (2-18) | .009 |
| Hospital admission time, days | 30 (14-51) | 45 (29-64) | 10 (2-18) | <.001 |
| In-hospital mortality, causes | ||||
| Multiorgan dysfunction | 9 (28) | - | 9 (75) | |
| Stroke | 2 (6) | - | 2 (17) | |
| Bleeding | 1 (3) | - | 1 (8) | |
| Patient destination | ||||
| Death with device | 7 (22) | 0 (0) | 7 (58) | |
| Weaning from device | 20 (62) | 15 (75) | 5 (42) | |
| Transplant | 5 (16) | 5 (25) | 0 (0) | |
ALT, alanine transaminase; APACHE II, acute physiology and chronic health evaluation II; AST, aspartate transaminase; BMI, body mass index; CABG, coronary artery bypass surgery; ECC, extracorporeal circulation; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; HR, heart rate; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; MAP, mean arterial pressure; PaO2, arterial oxygen pressure; PCCS, postcardiotomy cardiogenic shock; RRT, renal replacement therapies; SAPS II, simplified acute physiology score; SOFA, sequential-related organ failure assesment score; TMCS, temporary mechanical circulatory support; VA, veno-arterial; VIS, vasoactive inotropic score; WBC, white blood cell count.
Continuous variables are expressed as mean±standard deviation or, if variables were not normally distributed, as median (interquartile range). Categorical variables are presented as frequency and percentage
Weaning from TMCS was achieved in 24 patients (77%). In 19 patients (61%), weaning followed myocardial function recovery, and in the remaining 5 (16%) a heart transplant was performed.
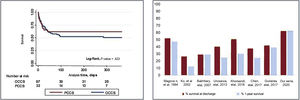
The survival rate at discharge of all treated PCCS patients was 63% (20 patients). There were no deaths during the first year after discharge. Survival at discharge and at 1-year of follow-up did not differ from that in patients with other causes of CS in our series (figure 1A). On univariate analysis, the main factors associated with in-hospital mortality were as follows: a history of diabetes or stroke, lactate levels, creatinine value 24 hours after ICU admission, peak creatinine value, glucose levels, highest value of procalcitonine during ICU stay, and acute neurologic complications (table 1). Most of these findings agree with those of previous series.1,3,4 On average, patients who died were 10 years older, but this finding did not reach stadistical significance probably due to the sample size.4
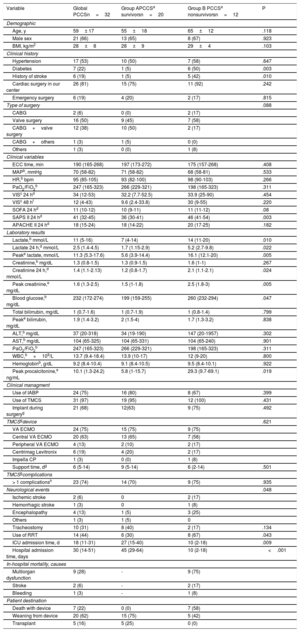
A: Kaplan-Meier analysis for 1-year survival estimates. Differences between postcardiotomy cardiogenic shock and other causes of cardiogenic shock in our series. B: comparison of survival at discharge and at 1 year in the main series collected recently by Lorusso et al., 1 including the results of our series. OCCS, other causes of cardiogenic shock; PCCS, postcardiotomy cardiogenic shock.
In recent years, due to an increased and generalized use of TSCM devices, the range of therapeutic possibilities available in PCCS has expanded. However, this development does not seem to have translated into a clear benefit in terms of hospital survival.1,2
This study shows some distinctive features of the experience of an organized CS unit, which reflect the contemporary management of PCCS in dedicated multidisciplinary teams. Despite the limited number of patients, which is common in CS series, the study shows one of the highest survival rates at discharge and at 1 year published to date (figure 1B). This experience could indicate the potential benefit of trained specialized teams operating within an organized structure,3 resulting in an immediate and probably more efficient response.5
Patients in our series showed tissue hypoperfusion and failure of other organs on ICU admission. Both conditions seem to improve by decreasing time to effective myocardial support with prompt use of an appropriate circulatory support system. Increases in hypoperfusion biomarkers were more significant in CS patients who died. However, the ranges that determine the prognosis and potential degree of reversibility of this damage are not yet well known.
Although ECMO seems to have become the first-line treatment as a TMCS, in our opinion, the use of other centrifugal central-access pumps should not be undervalued when uni- or biventricular failure is observed and respiratory support is not needed, especially when central access is available. The use of a peripheral access support in this context,1 which has the advantage of permitting sternal closure, did not seem to provide any further chances of survival in our series (table 1).
Another differential characteristic is the use of heart transplant as the final destination in 5 (16%) of the patients. This option has been less used in other series,1 and may suggest an easier access to emergency transplant in Spain, as opposed to the use of long-term assist devices.
Finally, this series confirms the excellent prognosis of CS patients who survive hospitalization. Thus, PCCS is a serious disorder with a high probability of early death, but it is treatable and, if appropriately addressed, can result in full recovery.
The limitations of our study include its observational nature and the limited number of patients involved. The applicability of our conclusions should be restricted to the clinical context described. Comparison between series remains challenging.3
We conclude that early detection of PCCS and rapid response by means of a dedicated, multidisciplinary and adequately organized shock team could improve management and survival in postcardiotomy shock patients. This conclusion should be confirmed in future series and lines of research.