To the Editor,
In recent years, left ventricular function has been shown to improve in patients with severe aortic stenosis treated with transcatheter aortic valve implantation (TAVI),1 and a reduction of left ventricular hypertrophy has also been reported,2 with the unquestionable prognostic implications this has over the medium term.
On the other hand, it has been demonstrated that right ventricular systolic dysfunction develops soon after aortic valve replacement surgery. However, few studies have evaluated right ventricular systolic function (RVF) in patients who had undergone TAVI, and these studies involved conventional echocardiographic methods, the results of which are subject to the limitations associated with these techniques.3, 4
Thus, our objective is to analyze the short- and medium-term course of RVF following TAVI using novel, more accurate echocardiographic methods that are less subject to technical limitations.5
This study had a case series design. RVF was quantified using the apical four-chamber view by means of tricuspid annular plane systolic excursion in M mode echocardiograms, tricuspid annular velocity was assessed by tissue Doppler imaging, and 2-dimensional speckle tracking echocardiography (2DSTE) was employed to determine 2 parameters: longitudinal strain in right ventricular free wall and tricuspid annular motion.5 Moreover, we analyzed the pulmonary artery systolic pressure using the tricuspid regurgitant jet velocity assessed by continuous-wave Doppler ultrasound, adding 10mmHg to the systolic pressure gradient between right ventricle and right atrium. Echocardiography (Philips iE33) was performed prior to, immediately after, and 1 and 6 months after implantation, and Philips QLAB software was employed to analyze the measurements obtained with 2DSTE.
The data for all the variables were collected prospectively. From June 2009 to September 2010, we enrolled 37 patients (age, 76 [7] years; 49% men; logistic EuroSCORE, 14.5% [5.9%]) with severe symptomatic aortic stenosis and high surgical risk, treated by means of percutaneous TAVI, with 6 months of follow-up. Informed consent had been obtained prior to inclusion.
Categorical variables were expressed as percentages, and continuous variables mean (standard deviation). The changes in continuous variables throughout follow-up were analyzed using repeated measures analysis of variance, and Bonferroni post hoc tests were performed when significant differences were found. The SPSS 18.0 statistical software package was employed. A P value less than .05 was considered to indicate statistical significance.
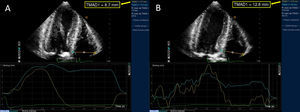
In the analysis of the results at hospital discharge, we observed a significant improvement in all the echocardiographic parameters associated with RVF, and the pulmonary artery systolic pressure was significantly reduced. However, the analysis of the results obtained at 1 month demonstrated a significant improvement only in novel echocardiographic parameters: tricuspid annular motion, from 17.6 (4.4)mm to 18.1 (4.8)mm (P<.001) (Figure); right ventricular strain, from −34 (4) to −36 (5) (P<.01). At 6 months, the results showed no significant further improvement in RVF in any of the parameters (Table). Seven of the patients in our study population required a permanent pacemaker; when they were excluded, there were no significant differences in any of the RVF parameters during the follow-up period.
Figure. Two-dimensional speckle tracking echocardiography showing tricuspid annular motion prior to (A) and 1 month after (B) transcatheter aortic valve implantation.
Echocardiographic Parameters of Right Ventricular Function Prior to Implantation, at Hospital Discharge and After 1 Month and 6 Months of Follow-up in Patients Who Had Undergone Transcatheter Aortic Valve Implantation
| Baseline | Discharge | P | 1 month | P | 6 months | P | |
| TAPSE, mm | 17.2 (2.5) | 17.6 (2.3) | .010 | 17.8 (2.4) | ns | 17.8 (2.6) | ns |
| TDI, cm/s | 12.2 (2) | 12.9 (1.7) | <.001 | 13 (1.6) | ns | 13.2 (1.8) | ns |
| TAM, mm | 15.6 (4.6) | 17.6 (4.4) | <.001 | 18.1 (4.8) | <.05 | 17.1 (7.3) | ns |
| RV strain | –29 (6) | –34 (4) | <.001 | –36 (5) | <.01 | –36 (5) | ns |
| PASP, mmHg | 48 (12) | 38 (9) | <.001 | 40 (6) | ns | 38 (8) | ns |
ns, not significant; PASP, pulmonary artery systolic pressure; RV strain, myocardial deformation measured by 2-dimensional speckle tracking echocardiography; TAM, tricuspid annular motion measured by 2-dimensional speckle tracking echocardiography; TAPSE, tricuspid annular plane systolic excursion using M mode echocardiography; TDI, tricuspid annular velocity measured using tissue Doppler imaging.
To the best of our knowledge, there are no studies in which RVF was evaluated in patients who had undergone TAVI using novel echocardiographic techniques based on 2DSTE. Our results show a very early improvement in RVF after implantation, in contrast to the findings after aortic valve replacement surgery, which is followed by a deterioration.3, 4 Forsberg et al.,3 who compared patients with surgical aortic valve replacement with patients who had undergone TAVI, report results similar to those obtained by us; however, Zhao et al.4 were unable to demonstrate any changes in RVF after TAVI. This discrepancy may be attributable to the fact that, in both studies, RVF was measured by tricuspid annular plane systolic excursion using M mode echocardiography, with the difficulties involved in aligning the ultrasound beam with the tricuspid annulus.5 However, the immediate improvement in RVF observed in our study, obtained with all the echocardiographic techniques, appears to confirm the positive results in terms of improvement obtained by Forsberg et al.3 On the other hand, 1 month later, further improvement in RVF is only observed with 2DSTE parameters, probably due to the greater diagnostic accuracy exhibited by these techniques in different clinical situations5 and their good correlation with the quantification of RVF by magnetic resonance imaging.6 There do not appear to be further improvements beyond the first month. Studies of larger series would help to determine whether there are other factors, such as pacing, that could influence RVF during follow-up. The small number of patients and the lack of hemodynamic data obtained during the follow-up period, for the purpose of assessing their relationship to the echocardiographic measurements, would be limitations to the study.
In conclusion, there is an early, and significant, improvement in RVF, quantified by all the echocardiographic techniques, and that 2DSTE parameters show a further improvement throughout the first month, possibly due to the greater accuracy of this technique for the quantification of changes in ventricular function.
.
Corresponding author: mpchiachio@hotmail.com