To the Editor,
Tako-tsubo syndrome is a disease with similar alterations to those observed in acute myocardial infarctions in the absence of coronary stenosis. Several pathophysiological mechanisms have been proposed to explain the unusual features of this syndrome, such as multivessel coronary vasospasm, abnormalities in coronary microvascular function, and catecholamine-mediated cardiotoxicity.1 Inflammation may also play a role, despite the fact that there is little evidence in the medical literature to relate inflammation with this syndrome.
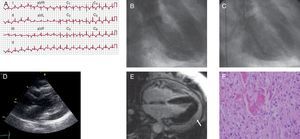
A 75 year-old woman with asthma and hypertension sought emergency care after an episode of oppressive retrosternal pain at rest, pain and symptoms related to the parasympathetic nervous system and no stress trigger. An electrocardiogram (ECG, Figure 1) showed 1mm ST-segment elevation in I, aVL, V1, and V2, and deep symmetrically inverted T waves. A physical examination revealed tachypnea with crackles in both lungs. An echocardiogram revealed anteroapical akinesia and moderate systolic dysfunction. Upon hospitalization, troponin I levels were 0.24ng/mL (reference value: up to 0.12ng/mL), with a peak of 1.74ng/mL within the first 24h, and normal creatinine kinase (CK). The coronary angiography did not reveal epicardial coronary lesions. The ventriculogram was consistent with extensive apical akinesia (Figure 1). The patient was admitted to the cardiology department, and was started on angiotensin-converting enzyme inhibitors and diuretics. After 72h, a control echocardiogram revealed moderate pericardial effusion, with no signs of haemodynamic instability, and she was administered non-steroidal anti-inflammatory drugs. A cardiac magnetic resonance imaging (MRI) performed 4 days after admission showed no pathological uptake in late gadolinium enhancement sequences and complete recovery of ventricular function, along with major pericardial effusion (Figure 1). We performed an echocardiogram that confirmed the presence of severe pericardial effusion, with findings indicating that the patient was hemodynamically compromised (Figure 1). We decided to drain the effusion by subxiphoid pericardiotomy and we also took a biopsy. A biochemical and haematological analysis of the pericardial fluid revealed elevated levels of lactate dehydrogenase, negative adenosine deaminase, and increased lymphocytes. The smear test and cell cultures were negative. The biopsy found pericardial thickening, with fibrin, histiocytes, and lymphocytes, which was consistent with a diagnosis of pericarditis (Figure 1). We completed the study by screening for autoimmunity and thyroid function, along with serology for hepatitis B and C, cytomegalovirus, Epstein-Barr, and adenovirus, and all tests were unremarkable. After removing the drain, the patient was discharged with anti-inflammatory treatment, and no further events occurred.
Figure 1. A: twelve-lead electrocardiogram with a significant ST-segment elevation in V1-V3, I, and aVL. B and C: ventriculogram (systole and diastole) consistent with extensive apical akinesia. D: echocardiogram (parasternal long axis view) showing severe pericardial effusion and diastolic collapse of the right ventricle. E: four-chamber magnetic resonance imaging showing no pathological uptake in the late enhancement sequence and pericardial effusion (arrow). F: pericardial biopsy revealing fibrin and infiltration with macrophages and lymphocytes (haematoxylin-eosin, ×400).
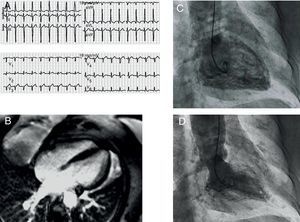
Two years later, the patient again sought emergency care for an episode of intense bronchospasms, which coincided with a house fire. She also had a feeling of retrosternal oppression. The patient was administered beta agonists on the ride to the hospital. Upon arriving, beta agonists were administered again, along with intravenous corticosteroids. An ECG (Figure 2) revealed sinus tachycardia, a left anterior hemiblock, and a 1mm ST-segment elevation in V2-V4. Troponin I was at 0.31ng/mL, with a peak of 0.89ng/mL, and CK was normal. An echocardiogram revealed mid-ventricular akinesia. We decided to admit her in the coronary unit, where she stayed for 48h. During hospitalization, urgent coronary catheterization was performed: coronary angiography found no coronary lesions, and a ventriculogram revealed mid-ventricular akinesia (Figure 2). After 72h in the hospital, we performed an MRI (Figure 2) that showed no pathological uptake and recovered ventricular function. She was discharged within 24h.
Figure 2. A: twelve-lead electrocardiogram showing 1mm ST-segment elevation in V2-V4. B: magnetic resonance imaging, late enhancement sequence, showing no pathological uptake in the myocardium. C and D: ventriculogram showing mid-ventricular akinesia.
Inflammation was proposed as the causal mechanism of tako-tsubo syndrome. In a study of MRI in 37 patients suspected of this syndrome,2 62% had inflammation along with pericardial effusion; however, other studies that involved a larger sample size did not reported effusion in association with this sydrome.3 Although the quantity of effusion was not specified, it was completely resolved spontaneously in all patients except one, and no patients needed a drain. Therefore, it is a very rare occurrence that pericardial effusion requiring invasive measures appeared in the sub-acute phase. To our knowledge, only one such case has been described4; however, this case of effusion had hemorrhagic characteristics in the context of high doses of sodium heparin, and a partially aborted coronary thrombosis could not be ruled out following the use of anticoagulants, with associated stunned myocardium and later recovery of ventricular function. Thus, the first episode suffered by the patient could have been an exceptional case of tako-tsubo, although we cannot completely rule out the diagnosis of myopericarditis. However, the probability that this was the true cause of the symptoms is low, given the patient's characteristics (female, age), the absence of fever, and the presence of effusion, as well as the serological and MRI results. On the other hand, recurrences in this syndrome are rare,5 approximately 10% of cases, and there is no identifiable triggering factors in most patients.6 It is unknown whether patients with associated pericardial effusion, where the level of inflammation is likely to be increased, have a greater tendency towards recurrence than those with less inflammation. In our case, the recurrence can probably be explained by adrenergic discharge generated by the mental stress of using beta agonists, which would involve a different pathophysiological mechanism than in the first episode, as evidenced by the different clinical evolution observed.
This case highlights the possible influence of inflammation in tako-tsubo syndrome, as well as the associated incidental appearance of severe pericardial hemorrhage, which would suggest recommending the inclusion of this syndrome in the differential diagnosis of pericardial hemorrhage. Furthermore, it suggests that certain patients exist with a myocardium that is susceptible to developing this syndrome through several different pathophysiological mechanisms.
Corresponding author: maapalomares@secardiologia.es