Cardiogenic shock (CS) occurs in approximately 5% of acute myocardial infarction cases and, despite improvements over the last few decades, it represents the leading cause of in-hospital mortality, remaining as high as 40%.1
MicroRNAs (miRNAs) bind target mRNA and act as posttranscriptional regulators of gene expression. MiRNAs can be detected in the circulation and have been proposed as promising biomarkers due to their robust stability to temperature changes and their resistance to degradation by endogenous RNase activity. More specifically, miR-21 is deregulated under cardiovascular disease conditions such as heart failure. MiR-122 has been reported to play a role in remodeling and fibrosis and in the early identification of ST-segment elevation myocardial infarction (STEMI) patients at higher risk of developing major adverse events after undergoing primary percutaneous coronary intervention (PCI), and has been found to be massively increased in a porcine model of CS.2,3 MiR-320a and miR-423-5p are highly expressed in the fetal heart.4 In addition, miR-320a is elevated in patients with chest pain of ischemic origin.5 MiR-423-5p has been validated in patients with heart failure and its abundance is related to disease severity.6
Therefore, in the present study, we sought to assess the expression dynamics of these miRNAs during the first 24 hours after patient admission to the unit. This prospective observational study included 51 consecutive patients with STEMI complicated with CS (approximately 4% of 1265 STEMI admitted), treated with primary PCI between February 2011 and March 2015. Six were excluded due to mechanical complications, and 2 did not sign the informed consent form and were not included; finally, 43 patients fulfilled the inclusion criteria. All-cause death at 30 days was 44.2% (n=19). In particular, inclusion criteria were systolic blood pressure <90 mmHg (or> 90mmHg with vasopressors for> 30 minutes), signs of poor peripheral perfusion, signs of pulmonary congestion and lack of rapid resolution after primary PCI. Postdischarge deaths were identified by telephone contacts and from electronic patient records.
Baseline demographics and clinical data were recorded during admission (Table 1). Serum samples were obtained at 3 time points (on admission immediately after primary PCI, at 12 hours, and at 24 hours), and stored at −80°C until analysis. Total RNA was extracted from 200μL of serum samples using miRCURY RNA Isolation Kit (Exiqon, Vedbaek, Denmark). A synthetic C elegans miRNA Cel-miR-39 (Qiagen, Hilden, Germany) was spiked in to normalize for extraction efficiency. Reverse transcription was performed with miScript II RT Kit (Qiagen) and quantification was performed using miScript SYBR Green PCR kit (Qiagen) and miRNA-specific miScript primer sets (Qiagen) on Roche LightCycler 480.
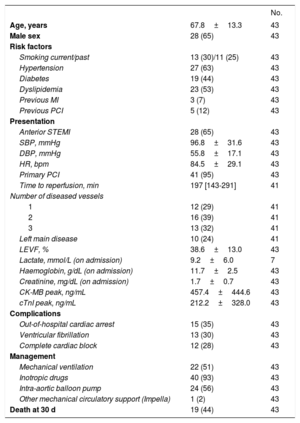
Demographic and Clinical Characteristics of the Studied Patients
| No. | ||
|---|---|---|
| Age, years | 67.8±13.3 | 43 |
| Male sex | 28 (65) | 43 |
| Risk factors | ||
| Smoking current/past | 13 (30)/11 (25) | 43 |
| Hypertension | 27 (63) | 43 |
| Diabetes | 19 (44) | 43 |
| Dyslipidemia | 23 (53) | 43 |
| Previous MI | 3 (7) | 43 |
| Previous PCI | 5 (12) | 43 |
| Presentation | ||
| Anterior STEMI | 28 (65) | 43 |
| SBP, mmHg | 96.8±31.6 | 43 |
| DBP, mmHg | 55.8±17.1 | 43 |
| HR, bpm | 84.5±29.1 | 43 |
| Primary PCI | 41 (95) | 43 |
| Time to reperfusion, min | 197 [143-291] | 41 |
| Number of diseased vessels | ||
| 1 | 12 (29) | 41 |
| 2 | 16 (39) | 41 |
| 3 | 13 (32) | 41 |
| Left main disease | 10 (24) | 41 |
| LEVF, % | 38.6±13.0 | 43 |
| Lactate, mmol/L (on admission) | 9.2±6.0 | 7 |
| Haemoglobin, g/dL (on admission) | 11.7±2.5 | 43 |
| Creatinine, mg/dL (on admission) | 1.7±0.7 | 43 |
| CK-MB peak, ng/mL | 457.4±444.6 | 43 |
| cTnI peak, ng/mL | 212.2±328.0 | 43 |
| Complications | ||
| Out-of-hospital cardiac arrest | 15 (35) | 43 |
| Ventricular fibrillation | 13 (30) | 43 |
| Complete cardiac block | 12 (28) | 43 |
| Management | ||
| Mechanical ventilation | 22 (51) | 43 |
| Inotropic drugs | 40 (93) | 43 |
| Intra-aortic balloon pump | 24 (56) | 43 |
| Other mechanical circulatory support (Impella) | 1 (2) | 43 |
| Death at 30 d | 19 (44) | 43 |
CK-MB, creatine kinase-MB; cTnI, cardiac troponin I 99th percentile cutoff value for the upper reference limit: 0.5 ng/mL; DBP, diastolic blood pressure; HR, heart rate; LEVF, left ventricular ejection fraction; MI myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction.
The study was approved by the Clinical Investigation Committee at Germans Trias i Pujol University Hospital and all participants, or their closest authorized relative, provided informed consent according to the declaration of Helsinki.
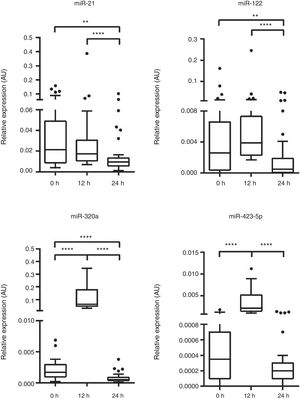
Analysis of the kinetics of the studied miRNAs revealed a significant increase in miR-320a and miR-423-5p at 12hours compared with baseline (P <.001). However, all studied miRNAs decreased significantly from 12 to 24 hours (P <.001). MiR-21, miR-122, and miR-320a levels decreased significantly to below baseline (P=.006, P=.003, and P=.000, respectively), whereas miR-423-5p levels in the plasma returned to baseline (P=.09; Figure 1).
In the presence of the multiorgan damage that characterizes CS, the origin of this boost in miR-320a and miR-423-5p at 12 hours is uncertain. Remarkably, the 4 studied miRNAs returned to baseline (or lower) after 24 hours despite the persistence of CS.
Although interesting, these results should be interpreted with caution. The limitations imposed by the small cohort, the small set of miRNAs under consideration, and the focus on STEMI patients should be taken into account, as other circumstances may reflect differently on these miRNA data.
Circulating miRNAs have generated strong expectations as emerging genetic prognosticators in addition to the current peptide bioarmamentarium. Studying the dynamics of these miRNAs, we have been able to dissect a pattern that could indicate an unknown role in CS.
In conclusion, monitoring of miR-21, miR-122, miR-320a, and miR-423-5p in STEMI patients with CS revealed dynamic behavior during the first 24hours after patient admission, with a peak at 12 hours.
FundingThis work was supported by grants from the Ministerio de Educación y Ciencia (SAF2014-59892), Fundació La MARATÓ de TV3 (201502, 201516), CIBER Cardiovascular (CB16/11/00403), CERCA Programme (Generalitat de Catalunya), the Helsinki-Uusimaa Hospital District, the Finnish Foundation for Laboratory Medicine, and the Liv och Hälsa Foundation.
We acknowledge Katariina Immonen for technical assistance.