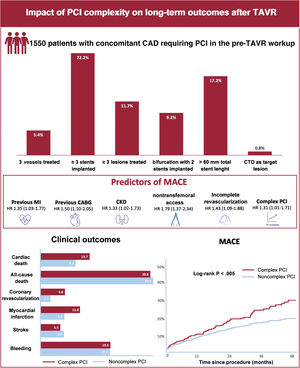
In patients undergoing percutaneous coronary intervention (PCI) in the workup pre-transcatheter aortic valve replacement (TAVR), the clinical impact of coronary revascularization complexity remains unknown. This study sought to examine the impact of PCI complexity on clinical outcomes after TAVR in patients undergoing PCI in the preprocedural workup.
MethodsThis was a multicenter study including consecutive patients scheduled for TAVR with concomitant significant coronary artery disease. Complex PCI was defined as having at least 1 of the following features: 3 vessels treated, ≥ 3 stents implanted, ≥ 3 lesions treated, bifurcation with 2 stents implanted, total stent length >60mm, or chronic total occlusion. The rates of major adverse cardiac events (MACE), including cardiovascular mortality, myocardial infarction, and coronary revascularization were evaluated.
ResultsA total of 1550 patients were included, of which 454 (29.3%) underwent complex PCI in the pre-TAVR workup. After a median follow-up period of 2 [1-3] years after TAVR, the incidence of MACE was 9.6 events per 100 patients-years. Complex PCI significantly increased the risk of cardiac death (HR, 1.44; 95%CI, 1.01-2.07), nonperiprocedural myocardial infarction (HR, 1.52; 95%CI, 1.04-2.21), and coronary revascularization (HR, 2.46; 95%CI, 1.44-4.20). In addition, PCI complexity was identified as an independent predictor of MACE after TAVR (HR, 1.31; 95%CI, 1.01-1.71; P=.042).
ConclusionsIn TAVR candidates with significant coronary artery disease requiring percutaneous treatment, complex revascularization was associated with a higher risk of MACE. The degree of procedural complexity should be considered a strong determinant of prognosis in the PCI-TAVR population.
Keywords
The prevalence of coronary artery disease (CAD) in the transcatheter aortic valve replacement (TAVR) population is fairly high, with up to 25% of TAVR candidates undergoing percutaneous coronary intervention (PCI) as a part of the preprocedural workup or at the time of the TAVR procedure.1 The need for coronary revascularization in TAVR patients remains controversial in such cases, due to the inconsistency of the available evidence, mainly based on nonrandomized data.2
Owing to the shared pathophysiology of aortic stenosis (AS) and CAD, TAVR recipients frequently exhibit multivessel disease and complex coronary lesions.3 Although the presence of concomitant complex CAD has been a common exclusion criterion in most randomized trials comparing TAVR vs surgical aortic valve replacement, evidence from observational studies suggests that the anatomical complexity of CAD and completeness of coronary revascularization might have an impact on the clinical outcomes following TAVR.4,5
Advanced CAD and a challenging subset of lesions usually translate into more complex revascularization strategies. In the TAVR setting, a complex coronary anatomy (SYNTAX score >22) has been associated with an increased risk of all-cause and cardiovascular mortality at 5-year follow-up.6 However, the impact of the complexity of percutaneous revascularization in this population remains largely unknown. Therefore, the aim of the present study was to evaluate, in a large cohort of patients undergoing PCI during pre-TAVR workup, the impact of PCI complexity on long-term outcomes after TAVR.
METHODSThis multicenter study included consecutive patients with severe AS and concomitant CAD who underwent a PCI as part of their pre-TAVR workup between 2007 and 2022. Data were derived from 15 centers in Canada, Europe, and Brazil. The study was approved by the ethics committee of each participating center and all patients provided informed consent for the procedures.
PCI was performed within 3 months before TAVR or at the time of the TAVR procedure. The management of CAD, including PCI strategy, the use of functional invasive tests for myocardial ischemia and intravascular ultrasound, and duration of antiplatelet therapy were left to the discretion of the physician responsible for the procedure. Significant CAD was assessed either by angiographic assessment (≥ 70% stenosis in an epicardial coronary vessel or ≥ 50% left main artery stenosis) or fractional flow reserve (≤ 0.80). Revascularization was considered complete when all significant lesions in vessels >2mm diameter had been successfully treated. The indications for TAVR, device type and procedural approach were assessed by each Heart Team based on an extensive clinical and anatomical preoperative assessment. The transfemoral approach was the default choice, and alternative access was reserved for patients with unfavorable iliofemoral anatomy.
Data collection and study definitionsBaseline, procedural, and follow-up data were prospectively collected in a dedicated database. Clinical follow-up was performed in each participating center at 1 and 12 months post-TAVR, and yearly thereafter, either by a medical visit or by telephone. The patient's vital status was updated at each medical contact, recording the date of the last contact for each patient.
Complex PCI included interventions with at least 1 of the following features: 3 vessels treated, ≥ 3 stents implanted, ≥ 3 lesions treated, bifurcation with 2 stents implanted, total stent length >60mm, or chronic total occlusion as target lesion.7 The information on the PCI procedure was obtained by procedural reports which were revised by 2 interventional cardiologists.
Clinical outcomesThe primary outcome was a composite of major adverse cardiac events (MACE), including cardiac death, nonprocedural myocardial infarction, and need for new coronary revascularization at follow-up. The secondary outcomes included the individual components of the combined primary endpoint, all-cause death, definite or probable stent thrombosis or restenosis, stroke, and bleeding. All adverse events were collected and adjudicated according to Valve Academic Research Consortium-2 criteria and acute coronary syndrome guidelines.8,9
Statistical analysisQualitative variables were reported as percentages and continuous data as mean±standard deviation or median (interquartile range [IQR]), depending on their distribution. Continuous variables were compared using the Student t-test (2-tailed) or Mann-Whitney U rank sum test, as appropriate. Qualitative variables were compared with the chi-square or Fisher exact tests. Survival curves were summarized using Kaplan-Meier estimates, and log-rank tests were used to compare groups. Cox multivariable regression analysis was performed to identify the independent predictors of MACE and all-cause and cardiovascular mortality in the whole PCI-TAVR cohort. Variables with clinical interest and with P<.10 on the univariable analysis were entered in a multivariable analysis. The multivariable analysis was performed using backward stepwise Cox regression. All analyses were performed using a hierarchical method to account for between-center variability. A 2-sided alpha level of 0.05 was used for all statistical testing. All statistical analyses were performed using SAS version 9.4 (SAS Institute, United States).
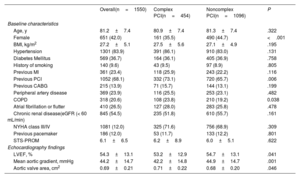
RESULTSA total of 1550 patients treated with PCI in the work-up pre-TAVR were included. Among them, 454 (29.3%) had at least 1 of the complex PCI criteria. Baseline clinical characteristics according to procedural complexity are reported in table 1. The mean age of the patients was 81.2±7 years, 42.0% were women, and the mean Society of Thoracic Surgeons Predicted Risk of Mortality score was 6.1% [3.1% to 6.9%]. Patients who underwent complex PCI were more frequently male and exhibited higher rates of previous PCI and chronic obstructive pulmonary disease.
Baseline characteristics according to PCI complexity
| Overall(n=1550) | Complex PCI(n=454) | Noncomplex PCI(n=1096) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 81.2±7.4 | 80.9±7.4 | 81.3±7.4 | .322 |
| Female | 651 (42.0) | 161 (35.5) | 490 (44.7) | <.001 |
| BMI, kg/m2 | 27.2±5.1 | 27.5±5.6 | 27.1±4.9 | .195 |
| Hypertension | 1301 (83.9) | 391 (86.1) | 910 (83.0) | .131 |
| Diabetes Mellitus | 569 (36.7) | 164 (36.1) | 405 (36.9) | .758 |
| History of smoking | 140 (9.6) | 43 (9.5) | 97 (8.9) | .805 |
| Previous MI | 361 (23.4) | 118 (25.9) | 243 (22.2) | .116 |
| Previous PCI | 1052 (68.1) | 332 (73.1) | 720 (65.7) | .006 |
| Previous CABG | 215 (13.9) | 71 (15.7) | 144 (13.1) | .199 |
| Peripheral artery disease | 369 (23.9) | 116 (25.5) | 253 (23.1) | .482 |
| COPD | 318 (20.6) | 108 (23.8) | 210 (19.2) | 0.038 |
| Atrial fibrillation or flutter | 410 (26.5) | 127 (28.0) | 283 (25.8) | .478 |
| Chronic renal disease(eGFR (< 60 mL/min) | 845 (54.5) | 235 (51.8) | 610 (55.7) | .161 |
| NYHA class III/IV | 1081 (12.0) | 325 (71.6) | 756 (68.9) | .309 |
| Previous pacemaker | 186 (12.0) | 53 (11.7) | 133 (12.2) | .801 |
| STS-PROM | 6.1±6.5 | 6.2±8.9 | 6.0±5.1 | .622 |
| Echocardiography findings | ||||
| LVEF, % | 54.3±13.1 | 53.2±12.9 | 54.7±13.1 | .041 |
| Mean aortic gradient, mmHg | 44.2±14.7 | 42.2±14.8 | 44.9±14.7 | .001 |
| Aortic valve area, cm2 | 0.69±0.21 | 0.71±0.22 | 0.68±0.20 | .046 |
BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; PCI, percutaneous coronary intervention; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality.
Data are expressed as mean±SD, n (%).
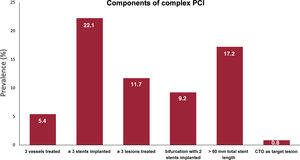
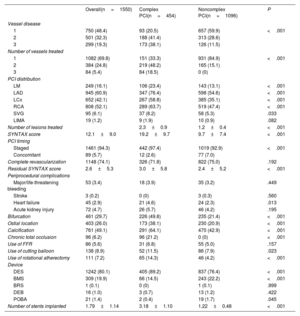
Procedural characteristics (for PCI and TAVR), and in-hospital outcomes of the study population stratified by PCI complexity are shown in table 2 and table 3. The median time between PCI and TAVR was 31 [8 to 68] days. Most (94.3%) PCI procedures were staged before TAVR, and a minority (5.7%) were performed simultaneously (at the same time as TAVR). Complete revascularization was achieved in 1148 (74.1%) patients. Procedural PCI features were largely unbalanced between complex and noncomplex PCI groups. Patients undergoing complex PCI exhibited more advanced CAD, as evidenced by a higher number of diseased and treated vessels, a higher baseline and residual mean SYNTAX score, a higher prevalence of chronic total occlusions, coronary calcification, ostial coronary lesions, and bifurcation lesions. The frequency of the complex PCI components in the overall population is shown in figure 1. Rates of periprocedural PCI complications did not differ between complex and noncomplex PCI, except for a higher occurrence of heart failure in the complex PCI group (4.6% vs 2.3%; P=.013).
Angiographic characteristics of coronary angiograms and PCI performed before TAVR according to PCI complexity
| Overall(n=1550) | Complex PCI(n=454) | Noncomplex PCI(n=1096) | P | |
|---|---|---|---|---|
| Vessel disease | ||||
| 1 | 750 (48.4) | 93 (20.5) | 657 (59.9) | <.001 |
| 2 | 501 (32.3) | 188 (41.4) | 313 (28.6) | |
| 3 | 299 (19.3) | 173 (38.1) | 126 (11.5) | |
| Number of vessels treated | ||||
| 1 | 1082 (69.8) | 151 (33.3) | 931 (84.9) | <.001 |
| 2 | 384 (24.8) | 219 (48.2) | 165 (15.1) | |
| 3 | 84 (5.4) | 84 (18.5) | 0 (0) | |
| PCI distribution | ||||
| LM | 249 (16.1) | 106 (23.4) | 143 (13.1) | <.001 |
| LAD | 945 (60.9) | 347 (76.4) | 598 (54.6) | <.001 |
| LCx | 652 (42.1) | 267 (58.8) | 385 (35.1) | <.001 |
| RCA | 808 (52.1) | 289 (63.7) | 519 (47.4) | <.001 |
| SVG | 95 (6.1) | 37 (8.2) | 58 (5.3) | .033 |
| LIMA | 19 (1.2) | 9 (1.9) | 10 (0.9) | .082 |
| Number of lesions treated | 2.3±0.9 | 1.2±0.4 | <.001 | |
| SYNTAX score | 12.1±9.0 | 19.2±9.7 | 9.7±7.4 | <.001 |
| PCI timing | ||||
| Staged | 1461 (94.3) | 442 (97.4) | 1019 (92.9) | <.001 |
| Concomitant | 89 (5.7) | 12 (2.6) | 77 (7.0) | |
| Complete revascularization | 1148 (74.1) | 326 (71.8) | 822 (75.0) | .192 |
| Residual SYNTAX score | 2.6±5.3 | 3.0±5.8 | 2.4±5.2 | <.001 |
| Periprocedural complications | ||||
| Major/life-threatening bleeding | 53 (3.4) | 18 (3.9) | 35 (3.2) | .449 |
| Stroke | 3 (0.2) | 0 (0) | 3 (0.3) | .560 |
| Heart failure | 45 (2.9) | 21 (4.6) | 24 (2.3) | .013 |
| Acute kidney injury | 72 (4.7) | 26 (5.7) | 46 (4.2) | .195 |
| Bifurcation | 461 (29.7) | 226 (49.8) | 235 (21.4) | <.001 |
| Ostial location | 403 (26.0) | 173 (38.1) | 230 (20.9) | <.001 |
| Calcification | 761 (49.1) | 291 (64.1) | 470 (42.9) | <.001 |
| Chronic total occlusion | 96 (6.2) | 96 (21.2) | 0 (0) | <.001 |
| Use of FFR | 86 (5.6) | 31 (6.8) | 55 (5.0) | .157 |
| Use of cutting balloon | 138 (8.9) | 52 (11.5) | 86 (7.9) | .023 |
| Use of rotational atherectomy | 111 (7.2) | 65 (14.3) | 46 (4.2) | <.001 |
| Device | ||||
| DES | 1242 (80.1) | 405 (89.2) | 837 (76.4) | <.001 |
| BMS | 309 (19.9) | 66 (14.5) | 243 (22.2) | <.001 |
| BRS | 1 (0.1) | 0 (0) | 1 (0.1) | .999 |
| DEB | 16 (1.0) | 3 (0.7) | 13 (1.2) | .422 |
| POBA | 21 (1.4) | 2 (0.4) | 19 (1.7) | .045 |
| Number of stents implanted | 1.79±1.14 | 3.18±1.10 | 1.22±0.48 | <.001 |
BMS, bare-metal stent; BRS, bioresorbable scaffold; DEB, drug-eluting balloon; DES, drug-eluting stent; FFR, fractional flow reserve; LAD, left anterior descending artery; LCx, left circumflex artery; LIMA, left internal mammary artery; LM, left main artery; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; RCA, right coronary artery; SVG, saphenous vein graft; SYNTAX, SYNergy between PCI with TAXUS and Cardiac Surgery; TAVR, transcatheter aortic valve replacement.
Data are expressed as n (%) or mean±SD.
TAVR procedural characteristics, and early outcomes according to the PCI complexity
| Overall(n=1550) | Complex PCI(n=454) | Noncomplex PCI(n=1096) | P | |
|---|---|---|---|---|
| Procedural characteristics | ||||
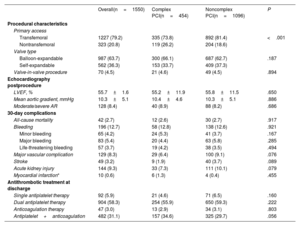
| Primary access | ||||
| Transfemoral | 1227 (79.2) | 335 (73.8) | 892 (81.4) | <.001 |
| Nontransfemoral | 323 (20.8) | 119 (26.2) | 204 (18.6) | |
| Valve type | ||||
| Balloon-expandable | 987 (63.7) | 300 (66.1) | 687 (62.7) | .187 |
| Self-expandable | 562 (36.3) | 153 (33.7) | 409 (37.3) | |
| Valve-in-valve procedure | 70 (4.5) | 21 (4.6) | 49 (4.5) | .894 |
| Echocardiography postprocedure | ||||
| LVEF, % | 55.7±1.6 | 55.2±11.9 | 55.8±11.5 | .650 |
| Mean aortic gradient, mmHg | 10.3±5.1 | 10.4±4.6 | 10.3±5.1 | .886 |
| Moderate/severe AR | 128 (8.4) | 40 (8.9) | 88 (8.2) | .686 |
| 30-day complications | ||||
| All-cause mortality | 42 (2.7) | 12 (2.6) | 30 (2.7) | .917 |
| Bleeding | 196 (12.7) | 58 (12.8) | 138 (12.6) | .921 |
| Minor bleeding | 65 (4.2) | 24 (5.3) | 41 (3.7) | .167 |
| Major bleeding | 83 (5.4) | 20 (4.4) | 63 (5.8) | .285 |
| Life-threatening bleeding | 57 (3.7) | 19 (4.2) | 38 (3.5) | .494 |
| Major vascular complication | 129 (8.3) | 29 (6.4) | 100 (9.1) | .076 |
| Stroke | 49 (3.2) | 9 (1.9) | 40 (3.7) | .089 |
| Acute kidney injury | 144 (9.3) | 33 (7.3) | 111 (10.1) | .079 |
| Myocardial infarction* | 10 (0.6) | 6 (1.3) | 4 (0.4) | .455 |
| Antithrombotic treatment at discharge | ||||
| Single antiplatelet therapy | 92 (5.9) | 21 (4.6) | 71 (6.5) | .160 |
| Dual antiplatelet therapy | 904 (58.3) | 254 (55.9) | 650 (59.3) | .222 |
| Anticoagulation therapy | 47 (3.0) | 13 (2.9) | 34 (3.1) | .803 |
| Antiplatelet+anticoagulation | 482 (31.1) | 157 (34.6) | 325 (29.7) | .056 |
AR, aortic regurgitation; LVEF, left ventricular ejection fraction: PCI, percutaneous coronary intervention; TAVR, transcathetr aortic valve replacement.
Data are expressed as mean±SD, n (%).
In TAVR procedures, transfemoral access was the approach of choice for most patients (79.2%). The adoption of nontransfemoral access was most frequent in the complex PCI group (26.2% vs 18.6%; P<.001). Balloon- and self-expanding transcatheter valves were used in 987 (63.7%) and 562 (36.3%) patients, respectively. No differences were observed between the 2 groups regarding postprocedural TAVR complications. At hospital discharge, 58.3% of patients received dual antiplatelet therapy, 34.1% were on an oral anticoagulant (monotherapy: 3.0%; in combination with antiplatelet therapy: 31.1%), and 5.9% received single antiplatelet therapy, with no differences between the complex and noncomplex PCI groups.
Long-term outcomes according to PCI complexityThe median length of follow-up of the study population was of 2 [1-3] years. A total of 82 patients (5.3%) were lost to follow-up, 486 patients (31.4%) died during the study period, 169 (11.1%) of them from cardiovascular causes.
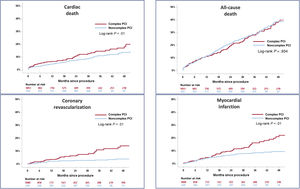
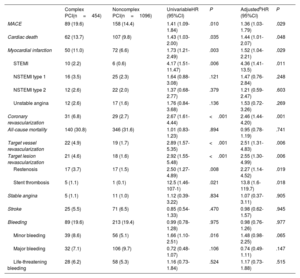
Long-term outcomes following TAVR stratified according to PCI complexity are summarized in table 4. The incidence of MACE (primary endpoint) was 9.6 events per 100 patients-years (hazard ratio [HR] for complex PCI, 1.31; 95% confidence interval [95%CI], 1.01-1.71; P=.042). The estimated event-free survival was 79.8 months in the complex PCI group vs 87.4 months in the noncomplex PCI group (P<.01). Patients in the complex PCI group had higher rates of cardiac death, myocardial infarction, coronary revascularization, target vessel revascularization, target lesion revascularization, restenosis, and stent thrombosis. Complex PCI was not associated with an increased risk of all-cause mortality, stroke, and bleeding events at adjusted analyses.
Long-term outcomes of the study population according to PCI complexitya
| Complex PCI(n=454) | Noncomplex PCI(n=1096) | UnivariableHR (95%CI) | P | AdjustedbHR (95%CI) | P | |
|---|---|---|---|---|---|---|
| MACE | 89 (19.6) | 158 (14.4) | 1.41 (1.09-1.84) | .010 | 1.36 (1.03-1.79) | .029 |
| Cardiac death | 62 (13.7) | 107 (9.8) | 1.43 (1.03-2.00) | .035 | 1.44 (1.01-2.07) | .048 |
| Myocardial infarction | 50 (11.0) | 72 (6.6) | 1.73 (1.21-2.49) | .003 | 1.52 (1.04-2.21) | .029 |
| STEMI | 10 (2.2) | 6 (0.6) | 4.17 (1.51-11.47) | .006 | 4.36 (1.41-13.5) | .011 |
| NSTEMI type 1 | 16 (3.5) | 25 (2.3) | 1.64 (0.88-3.08) | .121 | 1.47 (0.76-2.84) | .248 |
| NSTEMI type 2 | 12 (2.6) | 22 (2.0) | 1.37 (0.68-2.77) | .379 | 1.21 (0.59-2.47) | .603 |
| Unstable angina | 12 (2.6) | 17 (1.6) | 1.76 (0.84-3.68) | .136 | 1.53 (0.72-3.26) | .269 |
| Coronary revascularization | 31 (6.8) | 29 (2.7) | 2.67 (1.61-4.44) | <.001 | 2.46 (1.44-4.20) | .001 |
| All-cause mortality | 140 (30.8) | 346 (31.6) | 1.01 (0.83-1.23) | .894 | 0.95 (0.78-1.19) | .741 |
| Target vessel revascularization | 22 (4.9) | 19 (1.7) | 2.89 (1.57-5.35) | <.001 | 2.51 (1.31-4.83) | .006 |
| Target lesion revascularization | 21 (4.6) | 18 (1.6) | 2.92 (1.55-5.48) | <.001 | 2.55 (1.30-4.99) | .006 |
| Restenosis | 17 (3.7) | 17 (1.5) | 2.50 (1.27-4.89) | .008 | 2.27 (1.14-4.52) | .019 |
| Stent thrombosis | 5 (1.1) | 1 (0.1) | 12.5 (1.46-107-1) | .021 | 13.8 (1.6-119.7) | .018 |
| Stable angina | 5 (1.1) | 11 (1.0) | 1.12 (0.39-3.22) | .834 | 1.07 (0.37-3.11) | .905 |
| Stroke | 25 (5.5) | 71 (6.5) | 0.85 (0.54-1.33) | .470 | 0.98 (0.62-1.57) | .945 |
| Bleeding | 89 (19.6) | 213 (19.4) | 0.99 (0.78-1.28) | .975 | 0.98 (0.76-1.26) | .977 |
| Minor bleeding | 39 (8.6) | 56 (5.1) | 1.66 (1.10-2.51) | .016 | 1.48 (0.98-2.25) | .065 |
| Major bleeding | 32 (7.1) | 106 (9.7) | 0.72 (0.48-1.07) | .106 | 0.74 (0.49-1.11) | .147 |
| Life-threatening bleeding | 28 (6.2) | 58 (5.3) | 1.16 (0.73-1.84) | .524 | 1.17 (0.73-1.88) | .515 |
95%CI, confidence interval; HR, hazard Ratio; MACE, major adverse cardiac events; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as n (%).
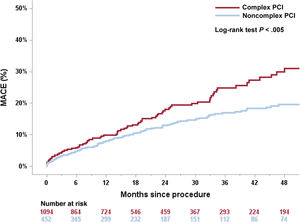
The Kaplan-Meier estimates for MACE at 4-year follow-up after TAVR are shown in figure 2. The 4-year follow-up MACE rates were 31.1% and 19.6% in the complex and noncomplex PCI groups, respectively (log-rank P<.005). Complex PCI significantly increased the risk for cardiac death, myocardial infarction, and coronary revascularization at 4-year follow-up (log-rank, P<.001). The complexity of PCI did not affect all-cause death rates (figure 3). Similar rates of bleeding events were observed, irrespective of PCI complexity.
Kaplan-Meier curves for MACE according to the PCI complexity. Kaplan-Meier estimates demonstrate higher rates of MACE (cardiac death, nonprocedural myocardial infarction, and need for new coronary revascularization) in the complex PCI group (red line) compared with noncomplex PCI (blue line) over 4 years of follow-up. MACE, major adverse cardiac events; PCI, percutaneous coronary intervention.
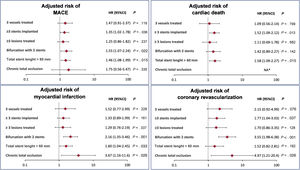
Adjusted risks for the cardiac ischemic endpoints (MACE and its individual components) according to the type of high-risk procedural features are shown in figure 4. PCI with ≥ 3 stents implanted and bifurcations with the 2-stent strategy were the angiographic subsets most strongly associated with increased ischemic risk.
Effect of high-risk procedural subsets on ischemic outcomes. Adjusted risk of MACE, cardiac death, myocardial infarction, and coronary revascularization among high-risk procedural subsets. 95%CI, 95%confidence interval; HR, hazard ratio; MACE, major adverse cardiac events; NA, not applicable. No cardiac death occurred in the chronic total occlusion group.
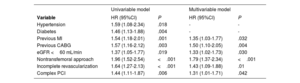
The risk of MACE following TAVR was 8.6%, 19.4%, and 29.7% at 1-, 3-, and 5-year follow-up. The univariable and multivariable analyses of the factors associated with the occurrence of MACE after TAVR are shown in table 5. In the multivariable model, previous myocardial infarction, previous coronary artery bypass graft (CABG), chronic kidney disease, nontransfemoral access, incomplete revascularization, and complex PCI were independently associated with an increased risk of MACE.
Independent predictors of major adverse cardiac events after transcatheter aortic valve replacement
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| Variable | HR (95%CI) | P | HR (95%CI) | P |
| Hypertension | 1.59 (1.08-2.34) | .018 | - | - |
| Diabetes | 1.46 (1.13-1.88) | .004 | - | - |
| Previous MI | 1.54 (1.18-2.01) | .001 | 1.35 (1.03-1.77) | .032 |
| Previous CABG | 1.57 (1.16-2.12) | .003 | 1.50 (1.10-2.05) | .004 |
| eGFR <60 mL/min | 1.37 (1.05-1.77) | .019 | 1.33 (1.02-1.73) | .030 |
| Nontransfemoral approach | 1.96 (1.52-2.54) | <.001 | 1.79 (1.37-2.34) | <.001 |
| Incomplete revascularization | 1.64 (1.27-2.13) | <.001 | 1.43 (1.09-1.88) | .01 |
| Complex PCI | 1.44 (1.11-1.87) | .006 | 1.31 (1.01-1.71) | .042 |
95%CI, 95% confidence interval; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The main findings of our study can be summarized as follows: 1) among patients referred for PCI in the pre-TAVR workup, about 1 out of 3 patients underwent complex coronary revascularization; 2) complex PCI was associated with higher rates of MACE after a median follow-up period of 2 years; 3) patients undergoing complex PCI exhibited a greater risk of cardiac death, coronary revascularization, myocardial infarction, and coronary thrombotic events, with similar rates of all-cause mortality; and 4) on multivariable analysis, PCI complexity emerged as independent predictor of MACE following TAVR, along with incomplete revascularization, previous myocardial infarction, previous CABG, nontransfemoral access and chronic kidney disease (figure 5).
Long-term outcomes of complex PCI in the TAVR population. Rate of complex PCI components in the PCI-TAVR population (top). Predictors of MACE following TAVR (middle). Clinical outcomes of the study population stratified according to PCI complexity (bottom, left), and Kaplan-Meier curve for MACE up to 4 years following TAVR (bottom, right). CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; CTO, chronic total occlusion; HR, hazard ratio; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement.
The management of concomitant CAD in TAVR candidates remains a largely unresolved issue. Although coronary revascularization in the preprocedural workup is routine practice in most TAVR centers, there is no evidence of its appropriateness, due to inconsistent data on the impact of CAD, its complexity, and the completeness of revascularization on outcomes.
Outside the TAVR population, several randomized trials failed to show any prognostic benefit of PCI in patients with stable ischemic heart disease.10 In the TAVR field, the only evidence from randomized studies has come from the ACTIVATION trial, showing that angiography-guided PCI pre-TAVR conferred no benefit in terms of reduced rates of mortality and rehospitalization at 1 year compared with conservative management, and, in contrast, increased bleeding risk.11 Nonetheless, the study was prematurely interrupted due to the slow recruitment rate and did not meet the formal noninferiority margin, thus precluding the drawing of definite conclusions on this topic. In addition, most of the patients included in that trial had single lesions and underwent simple PCI procedures. Moreover, a recent analysis investigated the impact of untreated chronic obstructive CAD on outcomes after TAVR, reporting relatively low rates of unplanned coronary revascularization and acute coronary syndrome at 1 year (0.7% and 0.5%, respectively),12 with a rise in incidence with increasing severity of CAD. Therefore, performing TAVR first in patients with concomitant significant CAD could be a safe option, and PCI could be considered post-TAVR in patients with residual symptom despite optimal medica therapy.
The presence of severe CAD represents an important factor that may influence the clinical decision-making process in AS patients. Patients with complex CAD were excluded from the randomized trials comparing TAVR and surgical aortic valve replacement, affecting the generalizability of the results to the general AS population.13,14 In a propensity-matched study, Alperi et al.4 showed that in patients with complex CAD (SYNTAX score >22 or unprotected left main disease), the percutaneous approach (PCI+TAVR) was associated with a similar risk of MACCE (all-cause mortality, myocardial infarction, coronary revascularization, and stroke) compared with a combined surgical intervention (surgical aortic valve replacement+CABG) after a median follow-up of 3 years. Nevertheless, a higher risk for repeat coronary revascularization was observed in TAVR+PCI recipients.
To date, few studies have investigated the impact of coronary lesion complexity on outcomes post-TAVR. In a study including 604 patients, the anatomical complexity of coexisting CAD was associated with a 2-fold increased risk of cardiovascular mortality after TAVR (SYNTAX score >22; HR, 1.84; P=.041).6 The presence of complex CAD emerged as an independent predictor for cardiac death, suggesting that it is not simply a marker of atherosclerotic burden, but rather a critical ischemic risk factor in AS patients. However, the study was limited by its small sample size, based on a single-center experience.
To the best of our knowledge, the present study is the first to show the impact of coronary revascularization complexity on long-term outcomes in a large, multicenter cohort of TAVR patients undergoing PCI during the preprocedural workup. This is noteworthy for 2 reasons: first, PCI complexity usually reflects that of CAD and, second, it provides a practical insight into the effect of revascularization techniques on clinical results. In our cohort, the complexity of PCI was significantly associated with poorer long-term outcomes. Having at least 1 of the complex PCI criteria conferred a higher risk of cardiac death, myocardial infarction, coronary revascularization, stent thrombosis, and restenosis after TAVR. Overall mortality did not differ between complex and noncomplex PCI patients. However, the baseline risk profile and the comorbidity burden, which mostly impacts the prognosis in TAVR patients, were similar among the 2 groups, which could explain the comparable outcomes in terms of all-cause mortality. In contrast, the differences were marked regarding the complexity of CAD and procedural features. About one-half of patients exhibited multivessel disease, and coronary lesions were frequently complex (B2/C type, calcified, ostial location), likely defining the excess cardiac mortality risk related to complex PCI. Moreover, the association between PCI complexity and ischemic risk is likely multifactorial. Patients undergoing more complex procedures usually have more advanced CAD, implying higher risk for atherothrombosis due to the natural progression of CAD.15 Treating more complex lesions (ie, bifurcations, calcified lesions) may increase the risk of incomplete stent apposition and delayed endothelialization, which may act as a trigger for platelet activation and subsequent intracoronary thrombosis.16,17
Our findings are in line with those previously reported in PCI studies, showing the harmful impact of procedural complexity.7,18,19 Giustino et al.7 showed that complex PCI was associated with a higher risk of ischemic but no bleeding events, also pointing out the protective role of prolonged dual antiplatelet therapy (≥ 1 year) in reducing the risk compared with a shorter dual antiplatelet therapy regimen. In a recent meta-analysis, PCI complexity increased the risk of both ischemic and bleeding events.19 Interestingly, in our study, the frequency of complex (vs simple) PCI was higher compared with previous PCI cohorts (29.3% vs 18%, respectively), as well as the rate of coronary events at follow-up, highlighting the pronounced high-risk profile of the TAVR population requiring PCI.7 However, it must be noted that the rates of stent thrombosis and restenosis after a median follow-up of 2 years were as low as 1.2 and 5.2%, respectively, providing reassuring evidence regarding the long-term results of pre-TAVR PCI.
Previous myocardial infarction, previous CABG, chronic kidney disease, and nontransfemoral access were identified as independent predictors of MACE after TAVR, and these results were in accordance with prior evidence reporting the detrimental effect of patient comorbidities after both PCI and TAVR.20 Of note, complex PCI and incomplete revascularization were also associated with an increased risk of MACE in the multivariable model. The clinical impact of revascularization completeness remains controversial. While some studies did not report a clear clinical benefit,21,22 revascularization incompleteness and residual SYNTAX score have been associated with poorer post-TAVR outcomes, supporting a complete revascularization strategy in the pre-TAVR workup.3,5 Randomized trials are needed to determine the strategy (conservative approach vs complete revascularization) that translates into the most favorable results after TAVR. Likewise, the potential role of physiologic assessment (fractional flow reserve, instantaneous wave-free ratio) in TAVR candidates should be elucidated. Even if physiology-guided PCI has been associated with better outcomes than angiography-guided PCI,23 data on the applicability of invasive functional testing in the AS setting are scarce, and warrant further validation of optimal threshold values.
Less invasive transcatheter treatments provide the opportunity to delay the management of either CAD or AS, balancing the sequence of the treatment according to patient's clinical status and centers’ practice. Nevertheless, considering the unfavorable impact of angiographic factors (number of diseased vessels), and procedural factors (stent number, stent length, bifurcation with 2 stent strategy), the revascularization strategy (whether and how) should be planned ahead and individualized. The decision to revascularize or not should be balanced according to the severity and location of coronary stenosis, patient's life expectancy, and risk of jeopardizing coronary reaccess after TAVR. How to revascularize remains more difficult due to the narrow tradeoff between avoiding procedural complexity and achieving complete revascularization.
The findings of this study highlight the central role of angiographic features and PCI complexity as an important risk factor for major adverse events that should be considered by the Heart Team when choosing the treatment strategy (regarding both AS and CAD) in patients with significant CAD. Further randomized studies are awaited to provide definitive evidence regarding the potential (un)benefit of routine revascularization in TAVR patients with stable ischemic heart disease and the prognostic meaning of the extent of myocardial revascularization.
LimitationsThis study has some limitations. First, the study design was observational and, although the data were collected prospectively, the present analysis was not prespecified and was retrospective. Although the risk for MACE was adjusted for relevant covariates, there might have been residual confounders due to unmeasured factors. The decision to perform PCI and the revascularization strategy were left to the discretion of the operator responsible for the procedure in each participating center, without predefined selection criteria. Even if data about the pharmacological regimen were prospectively collected, we were not able to perform a subanalysis evaluating the impact of different antithrombotic strategies on ischemic and bleeding outcomes, essentially due to the relatively small number of events at follow-up. In addition, almost one-third of our population required concomitant oral anticoagulation therapy, determining many possible therapeutic combinations. Finally, although clinical events were categorized according to standardized definitions, events were not adjudicated by an independent event adjudication committee.
CONCLUSIONSIn patients with severe CAD undergoing PCI in the pre-TAVR workup, those with complex PCI features exhibited poorer long-term outcomes, including a much higher risk for cardiac death, myocardial infarction and stent thrombosis up to the 4-year follow-up. In addition, PCI complexity emerged as an independent correlate of MACE after TAVR. Our study extends prior findings on complex PCI to the TAVR population, supporting the hypothesis that procedural complexity should be considered as an indicator for risk stratification during the pre-TAVR workup, which could potentially impact the treatment strategy. Further studies are warranted to better define the optimal management of CAD in patients with severe AS mainly concerning the need for routine revascularization, the extent of its completeness, and the type and duration of antithrombotic treatment.
FUNDINGJ. Rodés-Cabau holds the Research Chair Fondation Famille Jacques Larivière for the Development of Structural Heart Disease Interventions (Laval University).
ETHICAL CONSIDERATIONSThis study was conducted according to the ethics committee of each participating center, and all patients provided signed informed consent for the procedures. This study was conducted in accordance with the SAGER (Sex and Gender Equity in Research) guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools were used in the preparation of this study.
AUTHORS’ CONTRIBUTIONSM. Avvedimento and J. Rodès-Cabau conceived and designed the study. M. Avvedimento and J. Nuche merged local databases from all participating centers and were responsible for quality of data control. M. Côté performed the statistical analysis. M. Avvedimento wrote the first draft of the manuscript. All authors participated in the design and completion of local databases. All authors approved the final version of the manuscript and ensured the accuracy and integrity of the work. All authors had access to all the data in the study and had final responsibility for the decision to submit the manuscript for publication. J. Rodès-Cabau is responsible for the overall content of the study as guarantor.
CONFLICTS OF INTERESTJ. Rodés-Cabau has received institutional research grants and speaker/consultant fees from Edwards Lifesciences and Medtronic. The other authors report no conflicts with respect to the content of this manuscript.
- -
The prevalence of CAD in the TAVR population is fairly high, with up to 25% of TAVR candidates undergoing PCI as a part of the preprocedural workup or at the time of the TAVR procedure.
- -
TAVR recipients frequently have multivessel disease and complex coronary lesions.
- -
Previous evidence suggests that the anatomical complexity of CAD and completeness of coronary revascularization might have an impact on the clinical outcomes following TAVR.
- -
The degree of procedural complexity is a strong determinant of prognosis in the PCI-TAVR population.
- -
Patients undergoing complex PCI exhibit poorer long-term outcomes, including a higher risk of cardiac death, myocardial infarction, and coronary revascularization.
- -
In patients with significant CAD requiring coronary revascularization in the pre-TAVR workup, PCI complexity should be considered by the Heart Team as an indicator for risk stratification when choosing the treatment strategy.