Keywords
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a genetic cardiac disease with variable penetrance and heterogeneous clinical expression.1,2 Although several risk factors have been shown to be associated with increased risk for sudden death and progression to a dilated phase, the predictive accuracy of each of these adverse clinical markers is generally low. The mechanisms underlying sudden death and progression to a dilated phase are incompletely understood, but myocardial disarray, and myocardial fibrosis are thought to provide the anatomical substrate for ventricular arrhythmia, and left ventricular remodelling.3-11 Late gadolinium enhancement (LGE) with cardiac magnetic resonance, represents regions of increased myocardial fibrosis, and allows a direct assessment of the abnormal myocardial tissue.12-15 Previous studies, limited in sample size, showed that LGE extent is related to lower ejection fraction and progressive ventricular dilation and is associated with two or more clinical markers of sudden death.16,17
In an attempt to ascertain the clinical significance of LGE in HCM, we analyzed the relationship between the extent of LGE and left ventricular (LV) morphology and function, symptoms, functional capacity, and clinical risk factors for sudden death in a large cohort of patients with HCM.
METHODS
Patient Population
Over a 26-month period, cardiac magnetic resonance (CMR) studies were performed in 104 consecutive patients from our cohort of 360. We systematically ask for CMR studies in our patients with HCM, excluding patients with implantable cardioverter-defibrillators (ICDs), pacemakers, and atrial fibrillation because of difficulties in performing CMR. We also excluded for this study patients who underwent myectomy, alcohol septal ablation, or valve replacement prior to CMR and patients with known, or suspected coronary artery disease. HCM was diagnosed by the presence of a non-dilated and hypertrophied left ventricle (maximal wall thickness ≥ 15 mm in adult patients or ≥13 mm in adult relatives of a HCM patient) in the absence of another cardiac or systemic disease (eg, hypertension or aortic stenosis) capable of producing the magnitude of hypertrophy observed.11,18,19
Clinical Evaluation
Clinical evaluation included history, physical examination, 12 lead ECG, echocardiography, 24h ECG monitoring analysis, and conventional exercise testing, or exercise echocardiography with assessment of blood pressure, and ischemic response. HCM patients undergo comprehensive clinical assessments on an annual basis for risk stratification and evolution of symptoms, thus the time between the CMR scan, and the other procedures was, in general, no longer than a year.
Five clinical risk factors for sudden death were used to stratify patients: family history of sudden premature cardiac death; unexplained syncope; non-sustained ventricular tachycardia (one or more runs of ≥3 consecutive ventricular extrasystoles at ≥120 beats/min, lasting for less than 30 seconds); an abnormal blood pressure response during upright exercise testing in subjects ≤40 years old (failure of systolic blood pressure to rise by more than 25 mm Hg from baseline values or a fall of more than 10 mm Hg from the maximum blood pressure during upright exercise); and presence of severe left ventricular hypertrophy (wall thickness ≥30mm).
Of the 104 patients studied with CMR, all had two-dimensional and Doppler echocardiogram, 96 (92%) had 24h ECG monitoring analysis and 93 (90%) were assessed for an abnormal blood pressure response on exercise, 73 patients with exercise echocardiography and 20 patients with conventional exercise testing.
Cardiac Magnetic Resonance
Image Acquisition
All CMR images were obtained with a 1.5-T system (Gyroscan NT; Philips Medical Systems, Best, The Netherlands) in conjunction with a phased-array body coil and electrocardiogram gating.
Scout images were obtained in 3 orthogonal planes to determine the exact position and axis of the left ventricle. Cine-MR images of the left ventricle were obtained using a turbo gradient recalled echo sequence (repetition time ms/echo time ms, 11/4; flip angle, 20o; field of view (FOV), 400 mm; matrix, 147×256; section thickness, 10-mm, 1-mm gap between slices). The cine-MR sequences were obtained during expiration in the following planes: a short-axis view of the left ventricle from base to apex with eight to ten sections, one horizontal long axis view of the left ventricle, and one vertical long axis view in two left atrium-left ventricle chambers.
Myocardial tissue tagging gradient-echo echo-planar sequence (repetition time/echo time/echo-planar factor, 750/16/13 ms; flip angle, 13o; FOV, 400 mm; matrix, 102×256; slice thickness, 10 mm; orthogonal grid, 10 mm) was run during expiratory breath-hold and three short-axis planes (basal, midventricular, and apical) were obtained.
Delayed contrast-enhanced images were acquired 10 minutes after the injection of the contrast material according to a previous report,20 with an inversion-recovery T1-weighted sequence (repetition time/echo time ms, 8/4.5, flip angle 15o, FOV, 400 mm; matrix, 144×256; and section thickness, 10 mm) in 3 short-axis views taken at the base, midpapillary muscles, and the apex; 1 horizontal long-axis view; and 1 vertical long-axis view. The time of inversion was adjusted for each patient between 200 and 400 ms to achieve optimal suppression of normal myocardium.21,22 The CMR studies were completed on all patients without complications.
Image Analysis
All CMR images were analyzed on a satellite workstation console with commercial image post-processing software (EasyVision, version 4.0; Philips Medical Systems) by 2 radiologists with experience in cardiac MR imaging (RS and ER), whose joint opinion was reached by consensus. The CMR studies were read blinded to the clinical information.
The American Heart Association 17-segment model for the left ventricle23 was used to analyze wall thickness, contractile function, and delayed enhancement per segment. Three representative short-axis slices obtained at the base, mid-ventricle, and apex were divided into 6, 6, and 4 segments, respectively. The true apex (segment 17) was analyzed on the horizontal or vertical long axis of the left ventricle.
Endocardial and epicardial contours were traced manually at end-diastole and end-systole on the short-axis cine sets in order to measure left ventricular volumes, and calculate the LV mass, stroke volume, the ejection fraction, and the cardiac output.
Wall motion at rest was visually assessed as a change in myocardial tagging grip shape with respect to the original diastolic pattern. Circumferential segment shortening was judged as normal, hypokinetic, or dyskinetic in each myocardial segment.
Late gadolinium enhancement was considered present when the signal intensity of any area within the myocardium was highly hyperintense and persists in the same slice after swapping the phase encoding in order to exclude artifact images.
The patterns of LV hypertrophy by CMR were defined, as asymmetric when a ratio ≥1.3 of septum to free wall was present and apical when both an apical wall thickness ≥15 mm and a ratio ≥1,3 of maximum left ventricular short axis thickness at the apical level to the basal level were present.24
Exercise Echocardiography
Two-dimensional echocardiography using harmonic imaging was performed in standard parasternal and apical views, at baseline, peak exercise, and immediately after exercise.25,26 Peak exercise was defined when signs of exhaustion, ST depression >2 mm in the absence of chest pain, significant arrhythmia, severe hypertension (systolic blood pressure >240 mm Hg or diastolic blood pressure >110 mm Hg), severe hypotensive response (decrease >20 mm Hg from baseline), or limiting symptoms were present. The development of a new regional dysfunction or worsening from a previous hypokinetic region to akinesia was considered an ischemic response, as well as the decrease of LV ejection fraction >5% at the end of exercise.27,28 Differences of ejection fraction and subaortic gradient between basal, and peak exercise conditions were correlated with the number of segments with LGE. Exercise echocardiography assessment was performed by one investigator (J.P.), who was blinded to the clinical data.
Statistical Analysis
Data were analyzed using the SPSS software (version 12.0). Patients were classified in four groups on the basis of number of segments with late-enhancement: 0, 1, 2, or ≥3. The χ2 for trend was used to test for an association between these groups and each dichotomous baseline variable. Continuous variables were expressed as mean (SD) and associations were tested by linear regression. A P value less than .05 was considered significant.
RESULTS
Population Characteristics
There were 67 (65%) males and 37 (35%) women. Mean age at diagnosis was 43 years (12 - 76) and at the time of CMR imaging was 51 years (16 - 78). Most patients (98%) were in NYHA functional class I (50%) or II (48%); 19 (18%) reported exertional chest pain; and 8 (8%) previous syncope. Maximal left ventricular wall thickness on echo was 21 (6) mm (≥30 mm in 13 patients) and there was a subaortic gradient ≥30 mm Hg in 36 patients (35%). Family history of sudden death was present in 12 patients (11%), non-sustained ventricular tachycardia in 19 (18%), and abnormal blood pressure response on exercise test in 31 (30%). Forty-four patients had no risk factor for sudden death (42%), 42 (41%) had one, 14 (13%) had two, 3 (3%) had three, and 1 patient (1%) had four risk factors. Medical treatment used during follow-up included beta-blockers (65% of the patients), calcium antagonists (28%), disopyramide (3%), amiodarone (19%), acenocumarol (12%), ACE inhibitors (13%), and small doses of diuretics (20%). During the follow-up, 4 patients received an ICD (clinical data in Table 1), 4 alcohol septal ablation, and 1 patient underwent septal myectomy.
Cardiac Magnetic Resonance
The mean LV wall thickness was 14 (3) mm and the mean maximal wall thickness was 23 mm (range: 14 to 42 mm). The number of segments hypertrophied per patient ranged from 1 to 15 (mean: 6 [3]). Asymmetrical hypertrophy was present in 67 patients (65%), symmetrical in 25 (24%), and apical in 12 (11%). Hypertrophy was observed most frequently in anterior and posterior portions of the upper and middle septum.
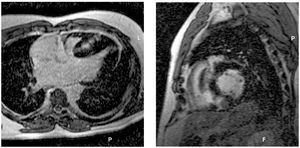
Late-enhancement was observed in 50 patients (48%), or in 131 segments (8%) out of 1768 segments, ranging from 1 to 11 segments per patient. Late-enhancement occurred most frequently within hypertrophied regions of the interventricular septum. The delayed enhancement patterns observed were: diffuse or confluent patchy mural foci (70%), subendocardial (10%), small foci (14%) and subepicardial (6%) (Figure 1).29 Although the majority of the hypokinetic segments presented LGE, of the 111 segments only 75 had LGE.
Figure 1. A 44 year-old man with non-obstructive hypertrophic cardiomyopathy, two risk factors for sudden death (non-sustained ventricular tachycardia and maximum wall thickness of 35 mm), LV ejection fraction of 65%, LV mass of 400 g, and 5 segments with late enhancement and hipokinesia at interventricular septum. A: horizontal long axis. B: short axis views on contrast-enhanced CMR images demonstrate confluent mural high signal intensity at the entire thickened septum.
Extent of Late Enhancement Related to Clinical Data
Table 2 summarizes the relation of LGE with age, symptoms, exercise test parameters, and risk factors for sudden death. There was no relationship between the extent of LGE and patients age at the time of CMR imaging (P=.3), but the number of LGE segments was inversely correlated with age at diagnosis (r=0.20; P=.04). With regard to sudden death risk factors profile, there was a higher proportion of wall thickness >=30 mm and non-sustained ventricular tachycardia (NSVT) with increasing the number of LGE segments (P<.001 and P=.04, respectively). There was a non-significant trend toward a higher proportion of two or more risk factors for sudden death with more LGE segments (P=.09). There were no significant differences in therapy and risk factors for coronary artery disease among LGE groups.
Two patients presented an ejection fraction <50% with a non-dilated LV, one with 2, and the other with 3 segments with LGE. The increase in subaortic gradient during exercise echocardiography correlated inversely with the number of LGE segments (r=0.26; P=.023). An ischemic response on exercise echocardiography was reported in 5 patients, and four of them had ≥3 segments with LGE (P=.003). Of the 5 patients with an ischemic response, 3 had angiographically normal coronary arteries and the other two patients did not have angina, or other symptoms indicative of coronary artery disease, and were reluctant to undergo a coronary angiography (one with a septal diffuse patchy LGE pattern and the other with a subepicardial pattern).
Table 3 shows the relation between LGE and other CMR parameters. The extent of LGE correlated positively with the LV maximum wall thickness (r=0.53; P<.001), the LV mass (r=0.41; P<.001) and the number of hypokinetic segments (r=0.51; P<.001), and inversely with the ejection fraction (r=0.32; P=.001) (Figure 2).
Figure 2. Relationship between the number of segments with late-enhancement and (A) the maximum left ventricular (LV) wall thickness, (B) LV mass, and (C) LV ejection fraction.
DISCUSSION
Myocardial fibrosis plays an important part in both sudden cardiac death and end stage disease as shown by necropsy studies, but its role in developing disease has not been established because of the lack of an in vivo quantification technique.11-14,30,31 Late gadolinium enhancement CMR provides a means of quantifying fibrosis in vivo in HCM and gives a new tool in order to better characterize the phenotype of this disease. This study, with the largest sample size of HCM studied with CMR to date, supports the clinical perspective that myocardial fibrosis, detected as LGE, may play an important role in disease expression.
LGE Related to Risk Factors for Sudden Death
Identifying patients at higher risk is an important aspect of the clinical management of HCM, particularly considering that effective preventive therapy is available (ICDs). The need for accurate risk stratification is challenging, taking into account that HCM patients who undergo ICD implantation are younger than patients with coronary artery disease and it is likely that their lifetime risk of serious ICD related complications will be high. LGE may potentially identify a substrate for increased risk for sudden death. In this respect, a previous study showed that greater extent of LGE was associated with two or more markers of risk for sudden death.17 We identified that among these clinical markers, the LGE extent is related to severe hypertrophy (>30mm) and NSVT. Late enhancement may be a potential link between these well known risk factors by which they relate to malignant ventricular arrhythmias.9,32 The fact that most patients with severe hypertrophy or NSVT did not die suddenly and that many sudden deaths occur in patients with a maximum wall thickness less than 30 mm or without NSVT, reflects the need for more accurate risk stratification and in these terms the extent of LGE may play a role. However, it is important to highlight that although the extent of LGE may relate to malignant ventricular arrthythmias, the presence of LGE in itself should not be considered as indicative of an adverse prognosis, since LGE is a common finding in HCM (50% in our study) and the overall risk of sudden death in our patient population is low (<1%). Moreover, the absence of LGE probably will not have a high negative predictive value. For example, one patient who received an ICD for primary prevention, presented multiple prolonged runs of NSVT and did not show LGE (Table 1). This may be explained by the fact that image contrast of LGE is created by suppressing normal myocardium and diffuse myocardial involvement can potentially be missed.21,22 On the other hand, the expression of this abnormal substrate is, in turn, influenced by factors such as autonomic tone and myocardial ischemia.9,33 In our study, an ischemic response during exercise echocardiography was not commonly observed, contrary to a previous dobutamine exercise echocardiography study,28 but its presence was linked to greater extent of LGE, which reflects another adverse clinical marker in this group of patients.
Although we did not find any association between the extent of LGE and patient age at the time of CMR scan, LGE extent was associated with an earlier diagnosis of the disease. This may imply that in some patients, extensive myocardial fibrosis does not need time to develop and large amount of fibrosis could be present at a young age. Moreover, the rate of LGE development may be important and extensive LGE at a young age may carry more significance than a similar degree of LGE in an older patient.
LGE Related to Systolic Dysfunction
Previous studies showed that LGE extent is associated with lower ejection fraction and with progressive ventricular dilation.16,17 Additional evidence that suggests a relation between the extent of LGE and systolic impairment is our finding of a positive correlation with the number of segments with hypokinesia and inverse correlations with the LV ejection fraction, and the capacity to increase subaortic gradient during exercise. The clinical course of end-stage phase in HCM proved to be variable, unpredictable, and generally unfavourable.34,35 Clinical markers that reliably anticipate evolution to systolic dysfunction are difficult to define in an heterogeneous disease like this. However, the extent of LGE with the other clinical features (young age at diagnosis, greater wall thickness, etc)34,35 may help us identify a subgroup of patients in which a close follow up may be warranted.
CONCLUSIONS
Late gadolinium enhancement has a great potential to provide new insights in the assessment of patients with HCM. The extent of LGE reflects a greater expression of this disease. It is associated with a more severe myocardial damage (lower ejection fraction and increased number of hypokinetic segments) and adverse clinical parameters (younger age at diagnosis, non-sustained ventricular tachycardia, severe hypertrophy, and ischemic response on exercise echocardiogram), suggesting it may be linked to prognosis. A follow up of this population will help us to evaluate the predictive accuracy of this technique for both sudden cardiac death and the development of systolic dysfunction.
Study Limitations
First, we estimated the extent of late enhancement in a semiquantitative way compared with the measurement of the volume of hyperenhancing lesions evaluated in previous studies.16,17 This methodological approach could explain the difference between our study and previous with regard to the strength of relationships observed between the extent of LGE and markers of clinical risk. However, this difference and the lower incidence of LGE in our study compared with previous may also be explained by different enrolling criteria and sample size. Choudhury et al studied only 21 HCM patients16 and Moon et al17 chose patients with high or low clinical risk factors for sudden death rather than taking a population "consecutively enrolled" as here. Our study confirms that the quantification of the extent of late gadolinium enhancement using the standarized myocardial segmentation model for tomographic imaging of the heart is clinically relevant and easily available for clinical application.36,37
Second, although all patients with documented coronary artery disease were excluded from this study, only patients with typical chest pain or symptoms indicative of coronary disease underwent coronary angiography, being possible that some patients with coronary artery disease were included. However, none of the patients with an ischaemic response on exercise echocardiography had a LGE pattern indicative of coronary artery disease.
ABBREVIATIONS
HCM: hypertrophic cardiomyopathy
CMR: cardiac magnetic resonance
LGE: late gadolinium enhancement
NSVT: non-sustained ventricular tachycardia
ICD: implantable cardioverter defibrillator
See editorial on pages 1-4
Dr Dumont was supported by the BBVA-Carolina Foundation.
Drs Monserrat, Fernández, Peteiro, and Castro-Beiras were supported by the Cardiovascular Research Network RECAVA-Instituto de Salud Carlos III. Dr Monserrat was supported by a research grant from Sanofi-Aventis Foundation.
Correspondence: Dr. Lorenzo Monserrat.
Servicio de Cardiología. Hospital Juan Canalejo.
Xubias de Arriba, 84. 15006 A Coruña. España.
E-mail: Lorenzo_Monserrat@canalejo.org
Received March 17, 2006.
Accepted for publication October 16, 2006.