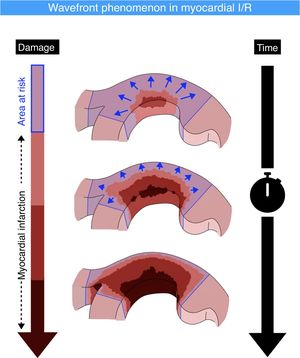
According to the wavefront phenomenon described in the late 1970s, myocardial infarction triggered by acute coronary occlusion progresses with increasing duration of ischemia as a transmural wavefront from the subendocardium toward the subepicardium. However, whether wavefront progression of necrosis also occurs laterally has been disputed. We aimed to assess the transmural and lateral spread of myocardial damage after acute myocardial infarction in humans and to evaluate the impact of metoprolol on these.
MethodsWe assessed myocardial infarction in the transmural and lateral dimensions in a cohort of 220 acute ST-segment elevation myocardial infarction (STEMI) patients from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction). The patients underwent cardiac magnetic resonance imaging at 5 to 7 days and 6 months post-STEMI.
ResultsOn day 5 to 7 post-STEMI cardiac magnetic resonance, there was a strong linear correlation between the transmural and lateral extent of infarction (delayed gadolinium enhancement) (r=-0.88; P<.001). Six months after STEMI, myocardial scarring (delayed gadolinium enhancement) was significantly less extensive in the transmural and lateral dimensions, suggesting that infarct resorption occurs in both. Furthermore, progression in both directions occurred both in patients receiving metoprolol and control patients, implying that myocardial salvage occurs both in the transmural and the lateral direction.
ConclusionsOur findings challenge the assumption that irreversible injury does not spread laterally. A “circumferential” or multidirectional wavefront would imply that cardioprotective therapies might produce meaningful salvage at lateral borders of the infarct.
This trial was registered at ClinicalTrial.gov (Identifier: NCT01311700).
Keywords
The wavefront theory of the progression of myocardial necrosis was first described in 1977 in a canine model of myocardial infarction.1 The theory, which is widely accepted, proposes that myocardial necrosis during acute coronary occlusion progresses with increasing ischemia duration as a transmural wavefront from the subendocardium toward the subepicardium, with no involvement of lateral extension.2,3 Nevertheless, the wavefront concept has been challenged by studies arguing that dogs have a well-developed system of epicardial coronary collaterals, thus making the subendocardium the most vulnerable myocardial region in this species. This critique takes into consideration the transmural heterogeneity of the left ventricular wall, which is evident from differences in myocardial blood flow, metabolism, contraction, and relaxation dynamics; compared with the subepicardial layer, the subendocardial myocardium has higher oxygen demand, myocardial pressure, and contraction strain.4 Moreover, Reimer and Jennings reported that infarct lateral margins in the subendocardial region are established within the first hour of coronary occlusion and are sharply defined by the anatomic boundaries of the ischemic bed at risk.5 This was subsequently supported by detailed anatomic studies demonstrating that intramural and epicardial collateral anastomoses in dogs and humans have end-capillary loops without microvascular connections between adjacent vascular beds; however, different findings were reported in other species.6 These findings supported a general view that there is no anatomic basis for a lateral extension of the border risk zone.
The wavefront theory triggered an explosion of studies assessing cardioprotective therapies.7,8 The theory established the idea that there is a large area of subepicardial myocardium in the ischemic bed that could be salvaged by early reperfusion but would otherwise die. This “theoretical” area at risk can include both infarcted (nonviable) and salvageable (viable) myocardium, setting the basis for the widespread use of early revascularization after acute ST-segment elevation myocardial infarction (STEMI).9 The aim of this approach is to avoid not only large infarcts, but also transmural scars, with both factors being associated with poor prognosis.2,10,11
Despite abundant evidence supporting exclusively transmural infarct wavefront progression, there are conflicting data regarding the species-dependent role of collaterals in the susceptibility of the subendocardium; moreover, some reports suggest a mismatch between the lateral border of subendocardial infarcts and the lateral border of the preexisting vascular bed of the occluded main coronary artery.12–14 These findings challenge the established “endo to epi” wavefront theory. A change in paradigm toward a circumferential wavefront would imply that cardioprotective therapies might produce meaningful salvage at the lateral infarct borders.
Here, we assessed myocardial infarction in the transmural and lateral dimensions in a cohort of acute STEMI patients from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction).15 Available cardiac magnetic resonance (CMR) data were assessed at an early stage after infarction (5-7 days post-STEMI) and a late stage (6 months post-STEMI). The aims of the present study were to a) describe the transmural and lateral spread of myocardial damage after acute myocardial infarction; b) evaluate the spatial behavior of the healing process in both dimensions; and c) assess the transmural and lateral infarct extension and evaluate the spatial behavior of the healing process in both dimensions by stratifying the cohort for their randomization arm (control vs metoprolol).
METHODSStudy population and interventionThe study population included patients enrolled in the METOCARD-CNIC trial (NCT01311700).15–18 METOCARD-CNIC recruited 270 patients with a first anterior wall STEMI and undergoing primary percutaneous coronary intervention. The patients were randomized to either receive intravenous (i.v.) metoprolol (up to 15mg) before primary percutaneous coronary intervention or conventional therapy (no i.v. metoprolol). The inclusion and exclusion criteria are reported elsewhere 15,16. All patients received oral metoprolol (first dose 12-24hours after reperfusion), irrespective of the arm to which they were randomized. CMR was performed in 220 patients at 1 week (5-7 days) and in 215 of the same patients at 6 months after STEMI. There were no between-group differences in demographic factors, cardiovascular risk profile, or procedural characteristics.15
Cardiac magnetic resonance data acquisitionA detailed description of the CMR protocol and methods of analysis is reported elsewhere.15,16 CMR imaging analyses were performed at the core laboratory at Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC). Data were acquired with 1.5 and 3.0 T CMR scanners. Steady-state free precession functional cine imaging was used to acquire the 2-, 3- and 4-chamber views and a stack of contiguous short-axis slices to cover the whole left ventricular (LV). Data acquisition parameters were as follows: voxel size, 1.6×2mm; slice thickness, 8mm; gap, 0mm; cardiac phases, 25-30; TR, 3.5; TE, 1.7; flip angle, 40; SENSE, 1.5; averages, 1; FOV, 360×360mm. For myocardial necrosis/fibrosis imaging, a segmented inversion recovery gradient echo sequence was acquired 10 to 15minutes after a cumulative dose of 0.2 mmol/kg intravenous gadolinium contrast agent (Magnevist, Schering AG, Germany).
Conventional cardiac magnetic resonance data assessmentCMR data were analyzed with dedicated software (QMass MR 7.5; Medis, Netherlands), as previously described.19 The extent of late gadolinium enhancement (LGE) was used as a surrogate for infarct size (necrotic region).20 LGE-positive areas were calculated according to the full-width-half-maximum methodology.15 End-diastolic LV volumes were measured.
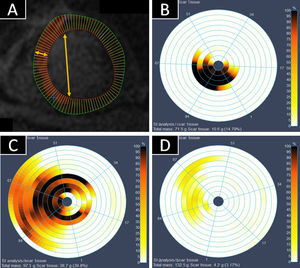
Cardiac magnetic resonance assessment of lateral and transmural infarct extentThe endocardial and epicardial contours of each LV short-axis slice were traced, and each contour was divided into 100 radial chords to allow a more granular evaluation (figure 1). Each chord traversed the myocardium and thus included subendocardium and subepicardium. Using this approach, infarct extent was characterized in its 2 dimensions: a) lateral (percentage of contiguous chords with LGE-positive myocardium in each slice); and b) transmural (percentage of enhancement within each chord). For the transmural dimension, 10% indicates involvement of only a small part of the chord (the immediate subendocardium) and 100% indicates involvement of the whole chord. All slices (and their associated percentages of transmural and lateral involvement) were weighted according to their relative mass to obtain a final mean percentage of lateral and transmural extent. Images were assessed and compared by evaluators blinded to treatment arm.
Assessment of transmural and lateral infarct extent by cardiac magnetic resonance (CMR). A: short-axis late gadolinium enhancement image showing the subdivision into 100 chords for subsequent analysis. Transmurality refers to myocardial involvement quantified from endocardium to epicardium (horizontal arrow). Laterality was measured as the number of chords in the infarcted area (vertical arrow). Each slice was weighted according to its mass. B-D: bulĺs eye charts of 3 representative infarcts on 5-day CMR. The color range from white to black represents the percentage of transmural necrosis in each chord. White indicates no late gadolinium enhancement (LGE), whereas black indicates complete transumural LGE. B: black chords indicate a transmural infarct. The involvement of few chords and slices indicate that the infarct is small. C: an infarct with lateral and transmural extension. D: a small nontransmural infarct.
The study was approved by the relevant ethics committees and institutional review boards at each participating center. All eligible patients gave written informed consent. All procedures performed in patients were in accordance with the ethical standards of each institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical methodsBaseline categorical characteristics are expressed as frequency (%) and were compared between groups using chi-square tests. Baseline continuous data are expressed as mean± standard deviation (SD) and were compared between groups by unpaired t test. Percentages of CMR lateral infarct extent were plotted against their corresponding percentages of transmural infarct extent, and the Pearson correlation coefficient was estimated to assess the strength of their association. Paired t tests were used for the longitudinal assessment (early vs late CMR) of lateral and transmural infarct extents.
The impact of metoprolol on lateral, transmural, and global myocardial infarct size was assessed with linear regression models, and treatment effect estimates and 95% confidence interval (95%CI) are presented without adjustment. Given the conceptual nature of the study, all analyses were performed according to the per-protocol principle (there were only 3 crossovers in this cohort).15
Differences were considered statistically significant at P <.05. All statistical analyses were performed in STATA version 15.1 (Stata Corp, College Station, United States). Some figures were produced in GraphPad Prism 6.00 (GraphPad Software, United States).
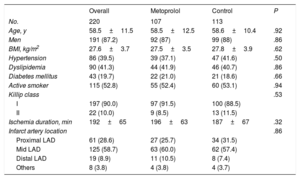
RESULTSStudy populationThe study included 220 patients with available CMR data obtained 5 to 7 days post-STEMI (107 who received metoprolol and 113 controls). Most study participants were men (87.2%), and there were high rates of cardiovascular risk factors: active smoking status (52.8%), dyslipidemia (41.3%), hypertension (39.5%), and diabetes mellitus (19.7%). There were no significant between-group differences in baseline characteristics and clinical presentation between groups (table 1) or in mean ischemia duration (196±63minutes in the metoprolol group vs 187±67minutes in the control group; P=.32).
Baseline patient characteristics by study arm
| Overall | Metoprolol | Control | P | |
|---|---|---|---|---|
| No. | 220 | 107 | 113 | |
| Age, y | 58.5±11.5 | 58.5±12.5 | 58.6±10.4 | .92 |
| Men | 191 (87.2) | 92 (87) | 99 (88) | .86 |
| BMI, kg/m2 | 27.6±3.7 | 27.5±3.5 | 27.8±3.9 | .62 |
| Hypertension | 86 (39.5) | 39 (37.1) | 47 (41.6) | .50 |
| Dyslipidemia | 90 (41.3) | 44 (41.9) | 46 (40.7) | .86 |
| Diabetes mellitus | 43 (19.7) | 22 (21.0) | 21 (18.6) | .66 |
| Active smoker | 115 (52.8) | 55 (52.4) | 60 (53.1) | .94 |
| Killip class | .53 | |||
| I | 197 (90.0) | 97 (91.5) | 100 (88.5) | |
| II | 22 (10.0) | 9 (8.5) | 13 (11.5) | |
| Ischemia duration, min | 192±65 | 196±63 | 187±67 | .32 |
| Infarct artery location | .86 | |||
| Proximal LAD | 61 (28.6) | 27 (25.7) | 34 (31.5) | |
| Mid LAD | 125 (58.7) | 63 (60.0) | 62 (57.4) | |
| Distal LAD | 19 (8.9) | 11 (10.5) | 8 (7.4) | |
| Others | 8 (3.8) | 4 (3.8) | 4 (3.7) |
BMI, body mass index; LAD, left anterior descending coronary artery.
Analysis performed according to per-protocol principle.
Data are presented as No. (%) or mean±standard deviation.
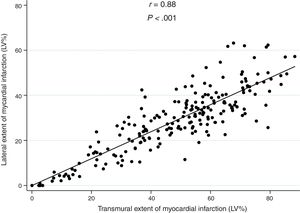
There was a strong linear correlation between transmural and lateral infarct size (r=0.88; P <.001; figure 2), which was maintained after adjustment for ischemia duration (P <.001). A similar association was observed across tertiles of ischemia duration: the lower tertile including shorter ischemia times (r=0.92; P <.001), the middle tertile (r=0.86; P <.001), and the upper tertile including longer ischemia times (r=0.86; P <.001).
Correlation between lateral and transmural infarct extent on early (5-7 day) cardiac magnetic resonance. There was a strong linear correlation between transmural and lateral infarct size (r=0.88, P <.001), and this was maintained after adjusting for ischemia duration (P <.001).LV, left ventricular.
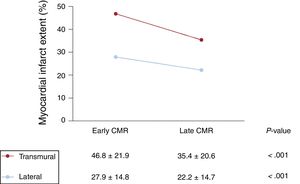
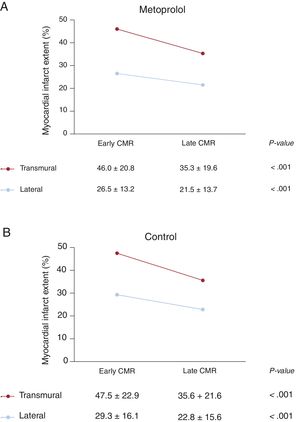
CMR studies were conducted at 6 months post-STEMI to allow analysis of the myocardial healing process in both dimensions between the early postinfarction stage (original 5–7-day CMR) and the late stage (6-month CMR). At 6-months post-STEMI, the infarcted myocardium was smaller in both dimensions (figure 3). Mean lateral infarct extent at 5 to 7 days and 6 months post-STEMI was 27.9±14.8% and 22.2±14.7%, respectively (P <.001). Transmurality also decreased significantly; mean percentage transmurality at day 5 to 7 was 46.8±21.9% and at 6 months 35.4±20.6% (P <.001). These parallel changes took place alongside an increase in LV end-diastolic volume from 171.5±36.0mL at 5 to 7 days post-STEMI to 190.6±42.8mL at 6 months post-STEMI (P <.001).
Evolution of cardiac magnetic resonance (CMR)-determined transmural and lateral infarct extent CMR studies were conducted serially to allow analysis of the myocardial healing process in both dimensions between the early postinfarction stage (original 5–7-day CMR) and the late stage (6-month CMR). At 6-month post-STEMI, the infarcted myocardium was smaller in both dimensions.
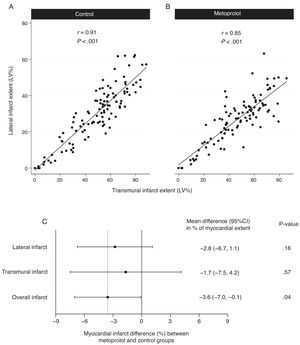
Consistent with previous results, transmural infarct size showed a direct linear correlation with lateral infarct size, both in the control group (r=0.91; P <.001; figure 4A) and the metoprolol group (r=0.85; P <.001; figure 4B). The smaller transmural and lateral infarct dimensions in the i.v. metoprolol group were consistent with the overall smaller infarct size in this group (figure 4C), although the statistical power was only sufficient to show significance for the effect on overall myocardial infarct size. At 6 months post-STEMI, infarct size had consistently reduced in both dimensions in both treatment arms. Differences in lateral and transmural scar progression between treatment arms are shown in figure 5.
Impact of i.v. metoprolol on cardiac magnetic resonance-determined lateral and transmural infarct extent. A, B: correlation between lateral and transmural infarct extent in the control and i.v. metoprolol patient groups. C: difference in infarct dimensions in the i.v. metoprolol group relative to control patients. 95%CI, 95% confidence interval; LV, left ventricular.
Our study shows that transmural and lateral extension of infarction after STEMI are closely correlated, suggesting that wavefront progression of a myocardial infarct takes place not only in the transmural direction, but also laterally. Moreover, the performance of early and late serial CMR studies reveals that myocardial healing progresses through infarct reductions in both the transmural and the lateral dimensions. Our findings also suggest that i.v. metoprolol, a proven cardioprotective therapy, seems to impede infarct progression in both dimensions. These findings challenge the classic view of unidirectional infarct wavefront progression (figure 6).
Our findings are consistent with the classic wavefront theory, which posits an initial necrotic zone in the subendocardial myocardium that progresses in the transmural direction toward the subepicardium, thus leaving the subepicardial myocardium as the ischemic bed salvageable by early reperfusion or other cardioprotective interventions.1,5 The transmural wavefront theory is supported by several observations: a) the ventricle shows transmural heterogeneity, with substantial differences in metabolism and hemodynamic properties (contractility, blood flow, pressure, etc.)21,22; the higher contractility demands and oxygen requirements of the subendocardium make this the most vulnerable region to ischemia; b) the coronary collateral circulation is distributed in the epicardium7,23; c) detailed anatomic studies in dogs and in humans have demonstrated that intramural and epicardial collateral anastomoses have end-capillary loops with no microvascular connections between adjacent vascular beds.6 However, while our findings do not contradict the transmural wavefront theory, the strong correlation found between transmural and lateral infarct extent in STEMI patients indicates the existence of a concomitant lateral wavefront, with extension of the infarct in one direction indicating extension in the other. Given the lack of serial CMRs in the acute postreperfusion phase, our study assumes that the extent of LGE, was used as a surrogate for myocardial infarct size, is relatively stable over time during the first week.
The existence of lateral wavefront progression is supported by increasing evidence. For example, the midmyocardium has been found to be the region most susceptible to ischemia in sheep, which have a limited system of preformed subendocardial coronary collaterals, making the subendocardial region the most resistant.21 Other studies have questioned the presence of “fixed” lateral boundaries in an ongoing myocardial infarction,12,13,24 describing a mismatch between the lateral border of postcoronary artery occlusion subendocardial infarcts and their preexisting vascular beds12–14; however, most of these studies were performed in animal models before the availability of current imaging technology or in human cohorts too small to support firm conclusions. More recently, van der Pals et al.25 used advanced imaging technology in a canine model to show that infarction transmurality at the edge of the area at risk measured by triphenyl tetrazolium chloride staining was less severe than that in the core. This suggested a potential lateral wavefront phenomenon, and the authors claimed that a lateral perfusion gradient exists within the midventricular perfusion territory of the occluded left anterior descending artery. The authors accurately quantified area at risk using a T1-weighted MOLLI sequence, obtaining excellent agreement with computed tomography perfusion imaging, myocardial blood flow measurements, and histopathology.25
The increase in LV end-diastolic volume between the early and late CMR can be explained as an adaption to the extra-load status in the infarcted heart.26–29 This change was paralleled by a decrease in infarct size in both dimensions when compared with early CMR. Although this finding suggests that the transmural infarct shrinks significantly over the 6-month period, the changes in the lateral dimension should be interpreted with caution given the general LV dilation. Currently it is not possible to determine whether this dilation is due exclusively to remodeling of the healthy myocardium or also reflects a contribution from myocardial healing in the lateral dimension.
Our findings are supported by the assessment of the impact of i.v. metoprolol on lateral and transmural infarct extension. Metoprolol is a well-established cardioprotective therapy,18,30 and the METOCARD-CNIC clinical trial was the first to compare 2 strategies of β-blocker administration/initiation in STEMI patients undergoing primary percutaneous coronary intervention: i.v. metoprolol before reperfusion vs postreperfusion oral metoprolol.15 The i.v. prereperfusion metoprolol strategy was associated with smaller infarcts,15 improved long-term left ventricular ejection fraction, and fewer cases of chronic severe LV dysfunction and consequent implantable cardioverter defibrillator placement.19 However, METOCARD-CNIC was underpowered to show effects on 1-year mortality or myocardial reinfarction.31 In the present study, stratification of METOCARD-CNIC CMR data by treatment group showed that metoprolol prevented infarct progression and promoted healing in both the transmural and the lateral dimensions, although this finding should be confirmed through formal statistical testing using interaction terms in an adequately powered study.
Study limitationsOur results have several limitations. Although we used an imaging technique with high spatial resolution, we lack a time series CMR scans to allow thorough assessment of necrotic wavefront progression. METOCARD-CNIC used edema as a potential surrogate for area at risk; however, recent studies have debunked the assumption underlying this strategy.20,32–35 The limitations of performing a CMR at a single time point after immediate reperfusion are that the edematous reaction within the first week is not stable and follows a bimodal pattern32–35 and that edema is also affected by cardioprotective therapies.20 Therefore, we were unable in this study to quantify myocardial salvage trends. Given the post hoc nature of the study, we were underpowered to detect differences between treatment groups in terms of reduction of the lateral and transmural necrotic areas when evaluated separately. Caution should be exercised when extrapolating our findings to nonanterior heart regions, although the observed histological changes are likely to occur regardless of STEMI location.36
CONCLUSIONSOur results show a close correlation between transmural and lateral extension of necrosis after myocardial infarction, strongly suggesting that wavefront progression occurs in both directions. Moreover, serial CMR at early and late postSTEMI stages shows that myocardial healing also operates in both the transmural and the lateral directions. Using a stratified analysis, infarct extension in both directions seems to be limited by prereperfusion administration of the established cardioprotective drug metoprolol.
FUNDINGMETOCARD-CNIC trial work was partially supported by the Centro Nacional de Investigaciones Cardiovasculares (CNIC), through CNIC Translational Grant 01-2009. Other sponsors were the Spanish Ministry of Health and Social Policy (EC10-042), the Mutua Madrileña Foundation (AP8695-2011), and a Master Research Agreement (MRA) between Philips health care and the CNIC. X.Rossello has received support from the SEC-CNIC CARDIOJOVEN fellowship program. R.Fernández-Jiménez has received funding from the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 707642, and is recipient of the ISCIII Fondo de Investigación Sanitaria grant No PI19/01704. BI is a recipient of the ISCIII Fondo de Investigación Sanitaria grants and ERDF/FEDER funds (PI16/02110, DTS17/00136, PI13/01979, SAF2015-71613-REDI) related to this topic. The CNIC is supported by the Ministerio de Ciencia, Innovación y Universidades (MICINN), the Instituto de Salud Carlos III (ISCIII), and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505).
CONFLICTS OF INTERESTThe authors have no conflicts of interest to disclose.
- -
The wavefront theory of the progression of myocardial necrosis was first described in 1977 in a canine model of myocardial infarction. This widely-accepted theory proposes that myocardial necrosis during acute coronary occlusion progresses with increasing ischemia duration as a transmural wavefront from the subendocardium toward the subepicardium, with no involvement of lateral extension
- -
The wavefront theory triggered an explosion of studies assessing cardioprotective therapies. The theory established the idea that there is a large area of subepicardial myocardium in the ischemic bed (area at risk) that could be salvaged by early reperfusion but would otherwise die.
- -
Our study shows a strong association between transmural and lateral extension of necrosis after myocardial infarction, strongly suggesting that wavefront progression occurs in both directions in the human heart. Moreover, serial CMR at early and late post-STEMI stages shows that myocardial healing also operates in both the transmural and the lateral directions.
- -
Our findings suggest that the administration of i.v. metoprolol, a proven cardioprotective therapy, impedes infarct progression in both dimensions.
- -
These findings challenge the classic view of unidirectional infarct wavefront progression and imply that cardioprotective therapies might produce meaningful salvage at lateral borders of the infarct.
The authors thank Carlos Galán-Arriola for help with the figure illustrating the multidirectional wavefront concept. S. Bartlett (CNIC) provided English editing.