Heart failure is a complex entity, with high morbidity and mortality. The clinical course and outcome are uncertain and difficult to predict. This document, instigated by the Heart Failure and Geriatric Cardiology Working Groups of the Spanish Society of Cardiology, addresses various aspects related to palliative care, where most cardiovascular disease will eventually converge. The document also establishes a consensus and a series of recommendations with the aim of recognizing and understanding the need to implement and progressively apply palliative care throughout the course of the disease, not only in the advanced stages, thus improving the care provided and quality of life. The purpose is to improve and adapt treatment to the needs and wishes of each patient, who must have adequate information and participate in decision-making.
Keywords
Cardiovascular diseases are the leading cause of death in Western countries.1 Most cases eventually converge in heart failure (HF), a condition characterized by progressive decline in the patient's functional status until death, which may also occur suddenly.2,3 This serious entity has high morbidity and mortality as well as an unpredictable prognosis, and end-of-life issues are often not addressed before advanced stages of the disease, when it is already too late.4 Furthermore, HF largely affects elderly patients, a group with frequent and serious comorbidities.3,5 One of the challenges faced by clinicians is providing adequate information that would allow patients and the people around them to understand the clinical course and prognosis of the disease.6
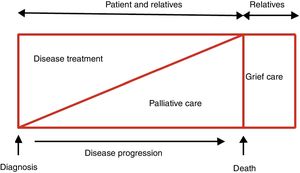
According to the World Health Organization, “Palliative care is an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual.”7 Palliative care (PC) was initially developed for cancer patients, but has since been recognized and implemented in other diseases associated with a poor prognosis, with cardiovascular disease currently accounting for up to 39% of persons requiring PC.7 This care should begin at the time of diagnosis, as the disease has a poor prognosis and is potentially fatal, becoming an increasingly pressing and relevant issue as the condition advances 2,8(figure 1). Additionally, care does not end with the patient's death, as it should be understood to also extend to the grief stage of family members and caregivers. All PC should be person-focused, acknowledging and respecting the needs and preferences of each individual while also seeking to improve and ensure access to the care needed, and can and should be provided at the various health care levels as well as at home. This document, backed by the Heart Failure and Geriatric Cardiology Working Groups of the Spanish Society of Cardiology, addresses the most important aspects related to PC in the field of HF.
Therapeutic objectives in patients with heart failure. Modified with permission from Martínez-Sellés et al.2
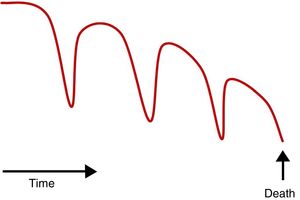
HF affects the quality of life of patients, as it causes them distress. 5,6The risk of death is high and rises with age, comorbidities, and hospitalizations.9 The clinical course is usually characterized by organ failure (figure 2), with the patient sometimes initially asymptomatic or “paucisymptomatic” and only experiencing a decline in functional status after an acute event (decompensation).2 Later, multiple rehospitalizations are not uncommon, and the patient exhibits progressive functional impairment, until death occurs due to a refractory situation and/or comorbidities.10 This impairment can also appear abruptly, although the increasingly widespread use of beta-blockers, mineralocorticoid-receptor antagonists, sacubitril-valsartan, and implantable cardioverter defibrillators (ICDs) means that fewer patients experience sudden cardiac death.3 The prognosis of the disease is hard to predict, and risk calculations are still imperfect, as they do not include prevalent conditions with prognostic impact,11 making them of limited use when deciding to revise the therapeutic approach (from “curative” to “palliative” treatment).12 Consequently, PC is not routinely included in the care process, adequate PC is received by only a small percentage of patients, and it is often provided at a late stage.13,14 In addition, patients with HF usually have a difficult course during the last year of life, with decompensations requiring multiple hospital visits. Death often occurs in the hospital, and a number of reasons make it difficult to take place at home, including family environment and lack of health care assistance to ensure adequate symptom control.15 Essential steps should include talking with the patient and relatives about the prognosis and potential course of the disease and planning the patient's care at the various stages. Therefore, it is crucial to know the patient's preferences for end-of-life care, such as the place where he or she wishes to be attended or prefers to die. These preferences may change over time and, along with advance directives and advance decision plans, are helpful for decision-making.16 Clinical trials, such as the PAL-HF study, have shown that interdisciplinary PC intervention in patients with advanced HF is more beneficial than conventional treatment in terms of quality of life, anxiety, depression, and spiritual well-being.17 Recent meta-analyses confirm the usefulness of including PC in improving the care and quality of life of patients with HF.18 There are still many PC-related issues and aspects to improve in this context, some of which are listed in .
MEDICAL TREATMENT. SYMPTOM CONTROLPatients with advanced HF require a review of drug indications, with particular focus on ensuring symptom control, avoiding adverse effects,19 and considering that a specific clinical manifestation may be the result of HF or its complications or comorbidities. Discontinuing medication is a personal decision that should be made by both the patient and his or her family.
The most common signs and symptoms in patients with advanced HF are dyspnea and systemic congestion, pain, depression, and asthenia.
DyspneaDyspnea is present in more than 60% of patients and is usually very disabling,20 and it may be associated with tachypnea and labored breathing. The main cause is pulmonary congestion, but it is necessary to rule out the presence of pleural effusion, intercurrent processes (eg, respiratory infections), and decompensation of comorbidities, which would require specific treatment. The therapeutic approach includes oxygen therapy, optimization of diuretic and vasodilator treatment, ambulatory inotropes, opiates, and, in certain situations, evacuation thoracentesis.
Oxygen therapyOxygen therapy is important in hypoxemic patients, but can also improve dyspnea even when the patient is not desaturated. There are other simple and effective measures, such as fresh air (breezes or fans) directed toward the patient's face.21
DiureticsDiuretics are the basis for treatment and are used for pulmonary decongestion. The route of administration and dosages should be consistent with the clinical situation and degree of congestion, with close monitoring of the patient's response so that the dosages can be adjusted when needed. In very advanced stages, an alternative may be to administer subcutaneous (s.c.) furosemide, both in the hospital and ambulatory settings,22 thus reducing hospitalizations.23 Conventional furosemide is alkaline (pH 8.5-9) and can cause skin irritation and, therefore, isotonic furosemide (pH 7-7.8) has been developed for s.c. administration, with a diuretic response similar to the intravenous (i.v.) route; however, it is not yet available on the market.24
VasodilatorsVasodilators can be used as support in the symptomatic treatment of dyspnea, in keeping with the usual recommendations for use in HF to prevent symptomatic hypotension.3
Ambulatory inotropesLevosimendan has shown advantages: for instance, its effects are sustained after the initial infusion, it can be used in patients treated with beta-blockers, and it does not increase oxygen requirements, among other benefits. The drug is used in patients with very symptomatic advanced HF despite optimal medical treatment, in order to alleviate symptoms, improve quality of life, and reduce rehospitalizations.25,26
OpiatesOpiates are indicated for the treatment of persistent dyspnea, and act on central and peripheral receptors, depressing the respiratory drive and enhancing patient comfort. They can be used safely, with no adverse impact on prognosis and with a low risk of respiratory depression if high doses are avoided.27 Morphine sulfate or morphine hydrochloride can be administered orally, and extended-release formulations are available.
Evacuation thoracentesisEvacuation thoracentesis is considered when there is significant pleural effusion.
Systemic congestionPeripheral edema is one of the most common signs in patients with advanced HF. Congestion can be treated with diuretics, but high doses and/or combined administration of several of them may be required; another safe and effective option in HF with refractory congestion is the use of hypertonic saline with high-dose i.v. furosemide.28 Lower limb edema can be particularly refractory to treatment, and certain therapeutic options should be encouraged, such as walking as much as possible, postural measures, and adequate treatment of the pain and of the ulcers and infections often also present.19
PainPain is common in advanced stages and is often underestimated.21 The cause is not well defined, but may be due to HF itself (hypoperfusion and ischemia, congestion), comorbidities (eg, degenerative osteoarthritis, diabetic neuropathy), or intercurrent processes. Regardless of the cause, any pain should be investigated, not minimized, and should be treated properly. First-line pain relief includes paracetamol and metamizole; nonsteroidal anti-inflammatories should be avoided due to their adverse impact on kidney function and water-electrolyte retention. In certain cases, colchicine can also be used as an anti-inflammatory agent. Adjuvant treatments can include antidepressants, antiseizure agents, benzodiazepines, or steroids (the latter with special care due to potential water retention). In a second step, first-line treatments and adjuvants are maintained and weak opiates (codeine, dihydrocodeine, tramadol) are added. If needed, a third step would then include the use of powerful opiates (morphine, fentanyl, buprenorphine, oxycodone, tapentadol).
DepressionA distinction should be made between depression and sadness and between anticipated pain and fatigue, insomnia, and other symptoms potentially caused by HF.29 Depression may respond to selective serotonin-reuptake inhibitors, although these drugs have been associated with hyponatremia and water retention, probably due to increased vasopressin.30 Tricyclic antidepressants should be avoided due to anticholinergic effects, orthostatic hypotension, and QTc prolongation, among other adverse effects.31 Treatment should be supplemented with supportive and nonpharmacological measures.2 Benzodiazepines are effective in patients with anxiety, but can contribute to asthenia.19
AstheniaAdvanced HF is associated with atrophy and osteomuscular weakness, which heighten fatigue and dyspnea.32 It is important to identify and treat secondary causes, such as anemia and iron deficiency, infections, and sleep disturbances. Physical training may be beneficial, but it is hard to apply in this context; it is recommended that patients maintain mobility and reduce bedrest as much as possible.21,32
Other symptomsIt is also important to treat insomnia, confusional syndrome, nausea, vomiting, anorexia, pressure ulcers, lower limb ulcers, changes in bowel rhythm, etc. The usual medications and measures for these symptoms in any context are also applicable. It is important not to minimize such symptoms, as they contribute to making the final stages of life more unpleasant and worsen the patient's perceived quality of life.19,21
PATIENTS WITH DEVICESPacemakers and defibrillatorsThe possibility to deactivate an implanted device over the course of the disease should be discussed with the patient and relatives at the time of implantation 4,33–35and afterwards, particularly if there are significant changes in disease progression (eg, rehospitalizations, ICD shocks), in order to avoid such conversations in the final days of life.4,36 It is essential to explain and document the advantages and disadvantages of each decision in the medical record, and the decision should be agreed among all professionals treating the patient.34
In the case of pacemakers, normal operation is recommended, as the devices do not affect the course of the disease and deactivating them can cause worsening of symptoms and even death in pacemaker-dependent patients.33 In patients receiving cardiac resynchronization therapy, biventricular pacing should be maintained due to the risk of worsening of symptoms.35 These devices should not be implanted or replaced in advanced stages of the disease.
A third of patients with advanced HF experience ICD shock therapy (appropriate or inappropriate) in the final stages of the disease, particularly in the last few days of life, causing pain and an unpleasant situation for the patient and family, and unnecessarily prolonging life.35 Deactivating these devices is widely considered to be an appropriate measure in this situation.33,34,36 It is also possible to deactivate antitachycardia therapies (retaining the “tracking only” mode) and antibradycardia therapies, particularly in pacemaker-dependent patients.4 If no programmer is available, the device can be disabled with a magnet.
Deactivation is also recommended in the case of other devices, such as subcutaneous event recorders or neurostimulators.35
Ventricular assist devicesLeft ventricular assist devices have helped improve the prognosis and quality of life of patients with advanced HF and, therefore, are increasingly used as destination therapy. It is recommended that consultation with PC specialists be routinely included in the patient assessment prior to implantation of these devices and that the required care be available at all times.37 However, less than half of patients have a consultation with PC specialists in the month prior to death, and most die in the hospital, in the intensive care unit, receiving advanced therapies such as mechanical ventilation or renal replacement therapy.38 Additionally, the decision to deactivate a ventricular assist device is an issue that should be jointly decided and should not be based on strictly medical criteria.
DO-NOT-RESUSCITATE ORDERDo-not-resuscitate (DNR) orders should offer patients, when duly informed, the possibility to voluntarily express prior refusal of cardiopulmonary resuscitation (CPR) in the event of cardiopulmonary arrest (CPA). Despite the prognosis of HF, the use of these DNR orders is less widespread than in other specialties, as they are used less frequently, at a later point in time, in a less convincing manner, and with more difficulty for interpretation.39
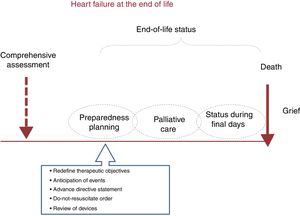
The development of a DNR is part of preparedness planning for end-of-life care (figure 3). This planning process allows patients to record their health care wishes and to participate in making relevant decisions about this stage of the disease. Conversations about preferences related to this care should be held before the end-stage, during outpatient follow-up visits, when greater clinical stability can be expected and the patient is able to actively participate in decision-making, or in the case of episodes of decompensation or ICD discharges.36
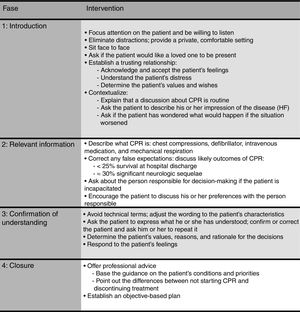
The entire process involves patients and encourages them to be properly informed of the situation and prognosis of HF from the time of diagnosis. Figure 4 summarizes the basic recommendations on the content and characteristics of conversations about DNRs: dialogue and questions should be encouraged to determine the patient's understanding of the condition and the meaning of CPA and CPR, but technical terms should be avoided.40
Basic instructions on the content and characteristics of conversations about do-not-resuscitate orders: guidelines for conversations. CPR, cardiopulmonary resuscitation; HF, heart failure. Modified with permission from Ruiz García et al.40
Patients tend to ask their physicians for professional advice and, consequently, the physician should be ready to answer appropriately based on the medical evidence and the preferences expressed by the patient. End-of-life conversations should conclude with a decision and an invitation to further discussions in the future to ensure that the patient's care plan will be in keeping with his or her wishes.41
Despite the benefit of approaching these conversations early, the vast majority of patients with HF acknowledge that they have not discussed end-of-life issues or resuscitation preferences with their physicians.16,39 In fact, only 12% of physicians involved in treating these patients acknowledge that they hold regular end-of-life conversations.42 The reasons include a lack of experience and training, a lack of communication skills and adequate vocabulary to explain the situation and prognosis in a comprehensible manner, an uncertainty about the progression of HF, a fear of causing unnecessary worry or hopelessness in early stages of the disease, and even a lack of time.42
A patient's excessive optimism can hinder the development and implementation of these DNR orders. Cardiologic patients’ perceptions regarding the prognosis of HF 43and CPR procedures 44is usually unrealistic. It is common for these patients to reject or to avoid asking for this type of information, in apparent hopeful ignorance, rather than to face a poor prognosis that offers no clear benefits. This overestimation of survival and recovery rates after CPR could influence their wishes and preferences regarding a treatment plan.44 Therefore, the patient needs early access to all comprehensible and up-to-date prognostic information, allowing him or her to adapt treatment strategies as desired at each stage of the heart disease.
In view of the above, it is necessary to stress the need to hold these conversations early on and to record the patient's wishes about CPR in the medical record to ensure they are clear and rapidly accessible. The DNR order should list the attending physician, the date of the decision, and a brief clinical summary of the reasons for the decision. This order, which can be amended at any time, should be clearly listed in the patient's medical record to ensure that all health personnel have rapid access to the information in case of CPR. The DNR decision should be shared at clinical sessions held with the rest of the multidisciplinary team in direct contact with the patient, as well as with the patient's primary care team.
Last, the DNR should only apply in the case of CPA, requiring that staff discontinue or refrain from starting CPR in this specific situation, but otherwise it should not affect care.39,41 In fact, it is hard to understand why drug treatments, diagnostic procedures, and nonpharmacological measures are underused in patients with HF who have a DNR in their medical record, compared with patients who do not.45
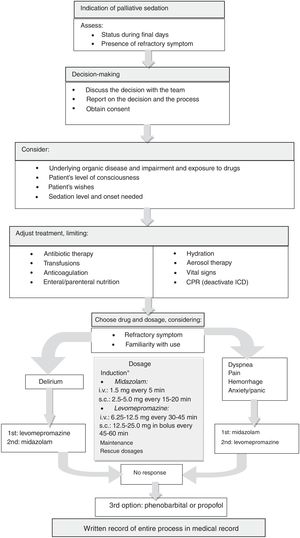
PALLIATIVE SEDATIONPalliative sedation should be considered a therapeutic tool to control refractory symptoms that have been unsuccessfully treated by other measures and that cause severe discomfort or suffering to the patient at the end of life.46 This measure consists of administering drugs, only at the necessary dosage, to reduce consciousness to the point where the patient no longer perceives the symptom,47 and requires patient consent. The process includes decision-making, record-keeping, procedures, and follow-up of the sedated patient (figure 5).
Palliative sedation process. CPR, cardiopulmonary resuscitation; ICD, implantable cardioverter defibrillator; i.v., intravenous route; s.c., subcutaneous route. *Until the required level of sedation is reached. The drug can be chosen based on the symptom: in case of dyspnea, pain or bleeding, midazolam is suggested; in case of refractory delirium, levomepromazine is initially recommended, although midazolam is also used on many occasions; midazolam can cause paradoxical agitation and has a maximum upper dose (around 160mg/24h), as levomepromazine also does (200-300mg/24h). If the maximum recommended dose is reached and the desired level of sedation has not been achieved with midazolam or levomepromazine, they may be combined, leaving propofol or phenobarbital as a final option (these last 2 drugs are used alone, not adjuvant to midazolam or levomepromazine).
Palliative sedation should be considered if:
- •
The patient is the final days of life.
- •
There are any refractory symptoms.
- •
The aim is to alleviate the suffering caused by this symptom.
- •
The patient desires it, has been duly informed, and consent has been granted by the patient or the designated person. When the patient cannot participate in the decision, the record of prior instructions should be consulted.
Because the decision may be complex, it should be made jointly by all professionals involved in the patient's health care whenever possible.
DocumentationThe entire decision-making process and procedure must be recorded in the patient's medical record.
Procedure- •
Any therapeutic effort should be adjusted. At the time, some treatments (anticoagulants, transfusions, and enteral or parenteral nutrition) are no longer indicated and should be discontinued. Likewise, the DNR order should be documented, and any ICDs should be deactivated.
- •
An adequate place should be found for the patient and his or her family, and any necessary care should be provided.
- •
Choice of drug. No specific drug has been shown to be superior over the others, but the drug used should meet the following requirements: rapid onset, easy dose titration, minimal adverse effects, and familiarity of use. Opiates are not indicated for palliative sedation,47 but can be used concomitantly if the refractory symptom is pain or dyspnea or if the patient was previously taking them. The following drug groups (and prototype) are those most commonly used in palliative sedation:
- –
Benzodiazepines: midazolam (used most often48).
- –
Neuroleptic agents: levomepromazine.
- –
Barbiturates: phenobarbital.
- –
Anesthetics: propofol.
- •
Calculation of the necessary dosage. It is also necessary to determine the induction dosage needed at the time to achieve the desired level of sedation from the initial level of awareness (figure 5).
- •
Maintenance dosage. If the aim is to continuously maintain the sedation level reached with the induction dosage, the necessary dosage will be administered as a continuous infusion. The induction dosage can be calculated by multiplying the number of doses that would have to be administered in 24 hours according to the mean half-life of the drug.
- •
Rescue dosage. When the patient's sedation level is temporarily inadequate, he or she may require a rescue dose, which will be the same dosage required for induction, except in the case of propofol, in which case it is 50% of the induction dosage.
The patient should be assessed regularly to check the level of sedation (the Ramsay scale can be used,49 see ), response to stimulation, temperature, bronchial secretions, and spontaneous muscle movements, and this should be noted in the medical record.
Adequate symptom control during sedation may require maintaining or adding other drugs.
ETHICAL CONSIDERATIONSCommunication and decision-makingCare for patients with advanced HF should comply with the 4 principles of bioethics,4 unless impossible because 2 or more principles are in conflict, in which case the care should be personalized. Patients should be encouraged to express and record their values, objectives, and preferences, particularly regarding end-of-life decision-making.4 In complex situations, a consultation should be considered with the health care ethics committee or the PC unit.36
Communication and early planning of care can often be improved. Preferences regarding life-support treatments are often not discussed, and in many instances DNR-related wishes are not recorded or differ from patients’ preferences.50 To improve this situation, open communication is essential from the time of diagnosis and should be enhanced at inflection points, such as hospitalizations, functional decline, or lack of further curative treatment options. Communication should be adapted to the patient's willingness to participate and should bring up the possibility of resuscitation and life-support treatments 51but also the goals and concerns of the patient and his or her relatives. These goals and preferences should be regularly updated. When appropriate, specific aspects of the disease should be discussed, such as fear of dyspnea and the protocols used to avoid it,52 ICD reprogramming,34 or ventricular assist disconnection.
Psychosocial and spiritual support to patients and families. Grief careAdvanced HF produces physical, psychological, emotional, and social distress, with repercussions for the person and for his or her family and surroundings.2 It is necessary to discuss the suffering and reactions to the illness, grief, sadness, discouragement, and depression experienced by the patient and his or her social and family environment. The concept of “person-focused care” is based on treating the patient as a whole person consisting of indivisible body, mind, and spirit. This concept acknowledges the importance of spirituality in a person's life (along with the physical and mental dimensions), an aspect often overlooked multidimensional aspect that deals with questions, values, beliefs about suffering and death, and religious and existential issues.53 Spiritual accompaniment should be understood as a professional and ethical approach. A therapeutic bond is formed through a relationship built on helpfulness and active listening, leading toward a peaceful death and leaving a wise and loving legacy to those left behind. The intervention consists of relieving and controlling symptoms and also of solving psychosocial problems to determine the needs, to dispel doubts, to encourage acceptance of the disease (coping), and to help finalize unresolved activities and emotional processes as well as social, family, and affective relationships, including preparation for death. In advanced HF, spiritual well-being remains stable and, although there is no clear equivalence with the symptomatic situation, experiencing spiritual peace is a better predictor of mortality than functional status and comorbidity, and stronger spiritual well-being is associated with a lower incidence of depression and, therefore, spiritual counseling has a positive impact on quality of life.54 Spiritual care should be integrated from a multidisciplinary perspective that should include, if the patient desires it, chaplains, pastors, or spiritual leaders from his or her religious persuasion. Regarding the family, proper intervention will involve identifying the main caregiver, assessing the patient's needs, understanding caregivers’ limitations or sense of overwhelming burden, providing support, enabling them to care for the patient, and in advanced situations preparing them for loss. Grief, in its various stages, has important psychological-affective consequences, which often require accompaniment. Grief counseling focuses on helping friends and family to express the pain and sorrow they feel about their loss. At this stage, the primary care physician and nurse and the PC team play a key role.
CONCLUSIONSHF is a complex clinical syndrome that leads to progressive and unrelenting functional decline in most cases and affects the patient's quality of life. Patients and families experience improved quality of life as a result of PC, and such measures should be considered from the time of diagnosis, and not merely in the final stages of the disease, with the patient actively informed and playing a key role in decision-making. It is essential to control symptoms and to improve quality of life, using an adequate therapeutic approach with drug-scaling and prioritizing patient comfort in terms of posology, route of administration and dosage, and psychosocial support. This care should also be offered to relatives during the grief stage. The involvement of the health authorities is also vital, as it ensures that PC is available to patients with HF.
CONFLICTS OF INTERESTNone declared.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.06.019