Transcatheter aortic valve implantation has experienced exponential growth in recent years.1 Aortic stenosis is frequently associated with coronary artery disease,2 and the performance of a coronary angiogram prior to implantation is indispensable. The need for preimplantation angioplasty is a matter of debate.3 In any case, given the increase in the rate of successful implantation and in the survival of these patients, it is becoming more common to treat patients with coronary artery disease who have received these prostheses.
The CoreValve® self-expanding prosthesis (Medtronic) is designed for placement above and below the origin of the coronary arteries. The purpose of this design is to ensure the patency of the coronary ostia. Its configuration in open cells theoretically allows intubation of the coronary arteries after implantation. On occasion, intubation of the coronary arteries can be difficult, since the imaging techniques usually employed do not have sufficient temporal and spatial resolution to determine the position of the cells with respect to the origin of the coronary arteries.
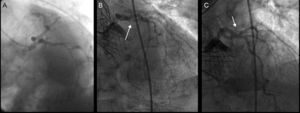
We present the case of a 75-year-old man who underwent implantation of a 26-mm CoreValve® prosthesis to treat symptomatic severe aortic stenosis and was at high surgical risk. Coronary angiography performed prior to implantation revealed no evidence of angiographically significant stenosis (Figure 1). Two years after implantation, the patient was admitted to the hospital with non-ST segment elevation acute coronary syndrome. Coronary angiography revealed 90% stenosis in the proximal circumflex artery (Figure 1), and the decision was made to perform angioplasty.
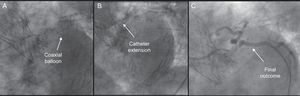
Left coronary artery was nonselectively intubated from a right radial approach through the cells of the prosthesis using a 4.0 6 Fr EBU guiding catheter, after attempting intubation unsuccessfully with 6 Fr AL1, 6 Fr AL2, and 3.5 6 Fr EBU catheters. A 0.014″ BMW guide wire was advanced, with difficulty, to the marginal branch; using a 1.2-mm coaxial balloon, it was exchanged for a high-support guide wire, over which a monorail catheter extension (Guideliner®, Vascular solutions, Inc.; Minneapolis, Minnesota, United States) was advanced. This provided enough support to advance and implant a 2.75mm × 18mm drug-eluting stent, with good angiographic results and no complications (Figure 2).
The performance of angioplasty in patients with self-expanding aortic valve prostheses has been reported elsewhere to be a safe and feasible technique; however, on occasion, it can prove to be a difficult and complex approach.4 The design of the CoreValve® prosthesis protects the origin of the coronary arteries and supposedly permits their intubation, but this can be complicated and nonselective, a circumstance that makes the performance of angioplasty extremely difficult due to the lack of support. The Guideliner® is a coaxial “mother and child” catheter mounted on a monorail system that extends the angioplasty guiding catheter and enables deep intubation of the coronary artery, thus providing extra support and improving coaxial alignment.5
To the best of our knowledge, this is the first report of a case in which a Guideliner® catheter was utilized for angioplasty following transcatheter aortic valve implantation with a self-expanding prosthesis. The use of extensions of this type may provide extra support in the performance of complicated angioplasties which, foreseeably, will be increasingly common in the near future.