Despite therapeutic hypothermia, unconscious survivors of out-of-hospital cardiac arrest have a high risk of death or poor neurologic function. Our objective was to assess the usefulness of the variables obtained in the early moments after resuscitation in the prediction of 6-month prognosis.
MethodsA multicenter study was performed in 3 intensive cardiac care units. The analysis was done in 153 consecutive survivors of out-of-hospital cardiac arrest who underwent targeted temperature management between January 2007 and July 2015. Significant neurological sequelae at 6 months were considered to be present in patients with Cerebral Performance Categories Scale > 2. An external validation was performed with data from 91 patients admitted to a third hospital in the same time interval.
ResultsAmong the 244 analyzed patients (median age, 60 years; 77.1% male; 50.0% in the context of acute myocardial ischemia), 107 patients (43.8%) survived with good neurological status at 6 months. The prediction model included 5 variables (Shockable rhythm, Age, Lactate levels, Time Elapsed to return of spontaneous circulation, and Diabetes – SALTED) and provided an area under the curve of 0.90 (95%CI, 0.85-0.95). When external validation was performed, the predictive model showed a sensitivity of 73.5%, specificity of 78.6%, and area under the curve of 0.82 (95%CI, 0.73-0.91).
ConclusionsA predictive model that includes 5 clinical and easily accessible variables at admission can help to predict the probability of survival without major neurological damage following out-of-hospital cardiac arrest.
Keywords
Out-of-hospital cardiac arrest (OHCA) is a major health problem, which affects between 76 and 110 patients per 100 000 inhabitants yearly. Despite the major efforts in treating these patients and the high costs involved,1 long-term prognosis remains poor, as two-thirds of resuscitated patients die before hospital discharge.2 Moreover, long-term mortality remains high after discharge, especially in patients> 65 years, of which nearly 1 in 3 die within the first year.3 In patients with OHCA due to ventricular arrhythmias who remain unconscious after their return to spontaneous circulation (ROSC), mild hypothermia has shown neuroprotective effects4,5 and its application has been extrapolated to survivors of cardiac arrest with initially nonshockable rhythms.6 Similar outcomes in patients treated with targeted temperature management (TTM) at either 33°C or 36°C have been reported.7 According to these data, TTM at a constant temperature between 32°C and 36°C for at least 24 hours is now recommended by international resuscitation guidelines.8 Despite TTM, neurological damage is frequent and plays a key role limiting the prognosis of these patients. Moreover, the need to maintain sedation and paralysis in this context lowers the accuracy of clinical examination and makes the determination of prognosis in the early moments after resuscitation a difficult task. Several studies had examined the accuracy of different diagnostic tests that can be performed after hospital admission, such as electroencephalogram findings,9 evoked potentials,10 brain imaging studies,11 and blood markers,12,13 or a combination of several parameters14–16 to estimate the prognosis for neurologic improvement in patients who are comatose after cardiac arrest. In this regard, current guidelines emphasize the need to wait to prognosticate a poor neurological outcome for a certain period of time after the return to normothermia, in patients who had undergone TTM, to minimize the rate of false-positive results.8 However, little is known about the prognostic value of variables available in the early hours, at the time of admission. In these patients, the initial decision-making may involve highly aggressive therapies, including extracorporeal membrane oxygenation17 and ventricular assist devices.18 In addition, this period of uncertainty, which can last for several days, also entails a significant emotional burden on families.19
Our objectives were to assess the association of the variables obtained in the early moments after resuscitation with 6-month prognosis and to generate and validate a predictive model to calculate the probability of survival without major neurological damage at 6 months.
METHODSSetting and Study PopulationA multicenter prospective study was performed in 3 academic hospitals in Spain. We included consecutive adult survivors of nontraumatic OHCA admitted to the intensive cardiac care unit who underwent TTM between January 2007 and July 2015 (only patients who survived until hospital admission and who underwent TTM where analyzed). The overall population was divided into 2 groups: data from patients admitted at 2 hospitals (Hospital General Universitario Gregorio Marañón and Hospital Universitario de Salamanca - derivation group) were used to create a predictive model, whereas data from patients treated at a third hospital (Hospital Universitario de Bellvitge - validation group) were used to conduct an external validation of the model.
Targeted Temperature ManagementAll survivors of OHCA with a Glasgow Coma Scale score ≤ 8 were admitted to the intensive cardiac care unit, monitored and treated according to international clinical standards and the institutional protocols, including adequate sedation, paralysis to prevent shivering during hypothermia, mechanical ventilation and, if needed, vasoactive or inotropic support was administered to maintain a mean arterial blood pressure> 80 mmHg. When indicated (briefly, if no obvious noncardiac cause was present or if ongoing ischemia was suspected), early coronary angiography and percutaneous coronary intervention were performed. In a subset of patients, hypothermia was started before hospital arrival, but in most patients, it was initiated as soon as possible after hospital admission. The target temperature was 32°C to 34°C, maintained for 24hours. Three methods were used: nonautomatic temperature control with thermal blankets and ice (12.0% of patients), and automatic temperature control systems, either with surface cooling device (in 79.3% of patients) or with an endovascular system (in 8.7% of cases). Rewarming was done gradually, avoiding hyperthermia. At the end of rewarming, sedation was stopped and fever was prevented.
Data Collection and OutcomesData were collected prospectively using Utstein-syle recommendations,20 from prehospital and in-hospital patient care records. We collected demographic information and other general characteristics including vital signs, whether OHCA was witnessed, and the presence or absence of bystander cardiopulmonary resuscitations. Time from collapse to first cardiopulmonary resuscitation (which will be approximate in patients with unwitnessed arrest), time from collapse to ROSC, and time from ROSC to initiation of therapeutic hypothermia were registered. Arterial blood sampling was performed on admission. Cardiac arrest was dichotomized as shockable rhythms (ventricular fibrillation and ventricular tachycardia) and nonshockable rhythms (including asystole and pulseless electrical activity). Postresuscitation circulatory shock was defined as the occurrence of arterial hypotension (mean arterial pressure <60 mmHg or systolic blood pressure <90 mmHg) sustained for more than 60minutes following hospital admission, despite fluid resuscitation, leading to noradrenaline administration. Neurological outcome was determined using the Pittsburgh Cerebral Performance Category (CPC),21 in patients who remained alive at discharge. This scale was assessed at hospital discharge and at 6 months. Data on neurological status at 6 months were collected from medical reports performed by physicians responsible for outpatient monitoring (who were blinded to model prediction results). The primary endpoint was survival with favorable neurological outcome at 6 months, defined as a CPC level of 1 (good recovery) or 2 (moderate disability), whereas CPC 3 (severe disability), 4 (vegetative state), and 5 (death) were considered as an unfavorable outcome.
As a general rule, the criteria for withdrawal of life-sustaining treatment in our centers is bilateral absence of N20 evoked potentials and/or and unreactive malignant electroencephalogram pattern, in a patient who remains comatose ≥ 72hours from ROSC and ≥ 48hours after rewarming.
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Statistical AnalysisContinuous variables are summarized as median and interquartile range, and differences were analyzed with the Student t test or with the Wilcoxon rank sum test (when not normally distributed). Categorical variables are presented as frequencies and percentages and were compared by the chi-square test or the Fisher exact test.
A multivariate logistic regression model was constructed with data from derivation group by backward stepwise variable selection. Variables with a P value of <0.2 in univariate analysis were considered as candidates for inclusion in the multivariate model. The Hosmer-Lemeshow test was used to determine goodness-of-fit. Possible medical relevant interaction terms between variables were tested. The screening accuracy of the model to identify poor outcome at 6 months (defined as death, vegetative state, or severe disability; CPC 3-5), from data available at hospital admission, was analyzed through odds ratio in multivariable logistic regression, the area under the curve (AUC), sensitivity-specificity coefficients, and the false-positive rate. The final model was evaluated, and external validation was performed using data from the validation group.
Data were collected locally, with removal of any identifying information, and were entered into a FileMaker database (FileMaker Inc, Santa Clara, California, United States). All tests were 2-sided and performed with Stata 12.1 (StataCorp, Lakeway, Texas, United States). Patients with a missing value in one of the variables of interest were reported and excluded from analysis.
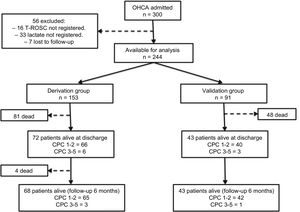
RESULTSStudy PopulationBetween January 2007 and July 2015, 300 patients were admitted to our institutions with a diagnosis of OHCA, persistent Glasgow Coma Scale score ≤ 8, and were treated with TTM. A total of 56 patients were excluded from the analysis: 33 had no lactate measurement at admission, 16 no information regarding time from collapse to ROSC, and 7 were lost to follow-up. Thus, 244 patients were available for analysis. As previously described, the population was divided into 2 groups: 153 patients in the derivation group, and 91 in the validation group.
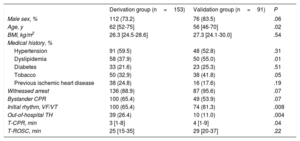
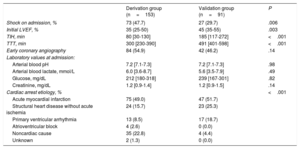
Patient Characteristics and OutcomesClinical features of the entire patient cohort are outlined in Table 1 and Table 2. There were several differences between the 2 groups: patients admitted in the derivation group were older, had less shockable rhythms, less cardiac etiology, and worse hemodynamic status at admission, a higher rate of out-of-hospital hypothermia, and shorter time to initiation of therapeutic hypothermia than those in the validation group.
Patient Characteristics at Baseline, Before Hospital Admission
| Derivation group (n=153) | Validation group (n=91) | P | |
|---|---|---|---|
| Male sex, % | 112 (73.2) | 76 (83.5) | .06 |
| Age, y | 62 [52-75] | 56 [46-70] | .02 |
| BMI, kg/m2 | 26.3 [24.5-28.6] | 27.3 [24.1-30.0] | .54 |
| Medical history, % | |||
| Hypertension | 91 (59.5) | 48 (52.8) | .31 |
| Dyslipidemia | 58 (37.9) | 50 (55.0) | .01 |
| Diabetes | 33 (21.6) | 23 (25.3) | .51 |
| Tobacco | 50 (32.9) | 38 (41.8) | .05 |
| Previous ischemic heart disease | 38 (24.8) | 16 (17.6) | .19 |
| Witnessed arrest | 136 (88.9) | 87 (95.6) | .07 |
| Bystander CPR | 100 (65.4) | 49 (53.9) | .07 |
| Initial rhythm, VF/VT | 100 (65.4) | 74 (81.3) | .008 |
| Out-of-hospital TH | 39 (26.4) | 10 (11.0) | .004 |
| T-CPR, min | 3 [1-8] | 4 [1-9] | .04 |
| T-ROSC, min | 25 [15-35] | 29 [20-37] | .22 |
BMI, body mass index; CPR, cardiopulmonary resuscitation; TH, therapeuthic hypothermia; T-CPR, time from collapse to first cardiopulmonary resuscitation; T-ROSC, time from collapse to return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Values are expressed as No. (%), or median [interquartile range].
Patient Characteristics After Hospital Admission and During Hospitalization
| Derivation group (n=153) | Validation group (n=91) | P | |
|---|---|---|---|
| Shock on admission, % | 73 (47.7) | 27 (29.7) | .006 |
| Initial LVEF, % | 35 (25-50) | 45 (35-55) | .003 |
| TIH, min | 80 [30-130] | 185 [117-272] | <.001 |
| TTT, min | 300 [230-390] | 491 [401-598] | <.001 |
| Early coronary angiography | 84 (54.9) | 42 (46.2) | .14 |
| Laboratory values at admission: | |||
| Arterial blood pH | 7.2 [7.1-7.3] | 7.2 [7.1-7.3] | .98 |
| Arterial blood lactate, mmol/L | 6.0 [3.6-8.7] | 5.6 [3.5-7.9] | .49 |
| Glucose, mg/dL | 212 [180-318] | 239 [167-301] | .82 |
| Creatinine, mg/dL | 1.2 [0.9-1.4] | 1.2 [0.9-1.5] | .14 |
| Cardiac arrest etiology, % | <.001 | ||
| Acute myocardial infarction | 75 (49.0) | 47 (51.7) | |
| Structural heart disease without acute ischemia | 24 (15.7) | 23 (25.3) | |
| Primary ventricular arrhythmia | 13 (8.5) | 17 (18.7) | |
| Atrioventricular block | 4 (2.6) | 0 (0.0) | |
| Noncardiac cause | 35 (22.8) | 4 (4.4) | |
| Unknown | 2 (1.3) | 0 (0.0) | |
LVEF, left ventricular ejection fraction; TIH, time from return of spontaneous circulation to initiation of therapeutic hypothermia; TTT, time from return of spontaneous circulation to target temperature.
Values are expressed as No. (%), or median [interquartile range].
Among the patients with an extracardiac cause (39 patients), a diverse group of etiologies was included: 15 were in relation to severe hydroelectrolytic disorders (mainly hypokalemia), 9 patients due to acute respiratory insufficiency of noncardiological origin, 6 patients in relation to acute cerebrovascular disease (all of them died during admission), 6 due to extensive pulmonary thromboembolism and, finally, 3 patients in whom OHCA was associated with cocaine intoxication.
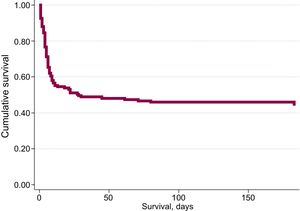
During admission, 129 out of the 244 patients (52.9%) died [81 patients (52.9%) in the derivation group and 48 patients (52.7%) in the validation group]. The most common cause of death was postanoxic brain injury (70.6%), followed by cardiogenic shock (18.8%), infectious complications (5.9%) and other causes in 4.7% of the patients. During the 6-month follow-up period, 4 additional patients died (all of them in the derivation group). Overall survival at 6 months was 45.5% (44.4% in the derivation group, and 47.3% in the validation group). Cumulative survival is shown in Figure 1. Regarding neurological outcome at 6 months, 107 of the 111 survivors (96.4%) had no significant neurological sequelae (98 patients with CPC=1, and 9 patients with CPC=2), while 4 patients had significant neurological damage (3 patients with CPC=3, and 1 patient with CPC=4). By groups, 65 of the 68 survivors in the derivation group (95.6%) and 42 of the 43 survivors in the validation group (97.7%) had no significant neurological sequelae (Figure 2).
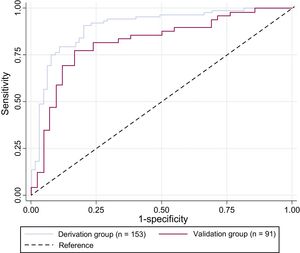
Prediction ModelIn the derivation group, 5 variables available at admission were associated with 6-month outcome (death or CPC> 2): Shockable rhythm, Age, Lactate levels, Time Elapsed to ROSC, and Diabetes (SALTED), Table 3. No significant interactions were found between the selected variables. We constructed a receiver operator curve (AUC, 0.90; 95% confidence interval [95%CI], 0.85-0.95) and calculate sensitivity (79.6%) and specificity (84.6%) for the prediction model, with a false-positive rate of 12.5%. This model demonstrated a good fit with a Hosmer-Lemeshow value of 0.97. When we applied this prediction model to the validation group, the receiver operator curve showed an AUC of 0.82 (95%CI, 0.73-0.91), with a sensitivity of 73.5% and a specificity of 78.6% (Figure 3).
Variables Available at Admission Independently Associated With an Unfavorable Outcome at 6 Months (Death or Pittsburgh Cerebral Performance Category> 2)
| aOR (95%CI) | P | |
|---|---|---|
| Nonshockable rhythm | 14.86 (3.96-55.79) | <.001 |
| Age (1 additional year) | 1.04 (1.01-1.07) | .02 |
| Lactate level (1 additional mmol/L) | 1.31 (1.09-1.58) | .004 |
| Time elapsed to return of spontaneous circulation | 1.04 (1.00-1.08) | .03 |
| Diabetes | 4.30 (1.40-13.16) | .01 |
95%CI, 95% confidence interval; aOR, adjusted odds ratio.
Receiver operator characteristic curves for predictive model using derivation and validation groups. The curves are based on diagnosis-prediction models of poor prognosis at 6 months incorporating 5 variables: Shockable rhythm, Age, Lactate levels, Time Elapsed to return of spontaneous circulation, and Diabetes (SALTED).
Although left ventricular ejection fraction at admission was significantly lower in patients with worse evolution (38.7% vs 43.1%; P=.01), this variable was eliminated from the multivariable logistic regression model during its development (backward stepwise variable selection).
The model was designed to estimate the long-term prognosis (6 months) and yet a high percentage of patients died during hospital admission (52.9%), which could be an important source of error. Therefore, the model was also applied to the subgroup of patients who survived at hospital discharge (115 patients). That analysis showed that the model maintained an adequate diagnostic power, with an AUC of 0.86 (95%CI, 0.79-0.93) ( and ).
Analysis of the etiology of cardiac arrest showed that patients with extracardiac origin had a much worse outcome (CPC ≤ 2 at 6 months in 12.8% compared with 49.8% in the remaining patients, P <.001). However, when the proposed prognostic model was applied to the subgroup of patients with extracardiac origin, the receiver operator curve maintained an AUC of 0.86 (95%CI, 0.71-1.00). The model was recalculated after exclusion of patients with an extracardiac origin, following the same principles described for the main model (in this case, derivation group of 118 patients and validation group of 87 patients). This subanalysis yielded fairly similar results to those of the main model, in which the same variables were maintained as independent prognostic predictors. The main difference was that the “nonshockable rhythm” variable became much more relevant in the estimation of prognosis: adjusted odds ratio 43.01 (95%CI, 39.43-47.08) vs 14.86 (95%CI, 3.96-55.79) in the main model. These characteristics of this model, as well as its behavior in external validation (AUC, 0.84; 95%CI, 0.75-0.93) when it was applied to the validation group, are detailed in .
DISCUSSIONThe present study, performed in comatose patients admitted after OHCA treated with therapeutic hypothermia, describes and validates a predictive model designed from variables obtained at admission to estimate the probability of survival without major neurological damage at 6 months. Overall survival to hospital discharge was 47%, whereas our cumulative survival with good neurological outcome at 6 months was 44%. These data are consistent with previous reported studies that include patients treated with an intensified postresuscitation approach (including TTM and early coronary angiography when indicated).4,5,7,13
We found that 5 readily available variables in the early moments after resuscitation can estimate the probability of survival without major neurological damage at 6 months: shockable rhythm, age, lactate levels, time elapsed to ROSC, and diabetes. Due to the complexity of calculating the predictive model based on the multivariate logistic regression coefficients, the estimation of the probability of an unfavorable outcome in each patient could be tedious in clinical practice, so we have designed a smartphone calculator based on FileMaker Go application called “SALTED”, which makes the calculation easy. Its calculation is only feasible when all the items are known, and the result is directly the estimated probability of having an unfavorable outcome in each specific patient.
This is not the first attempt at designing a model to predict prognosis after OHCA.22–32 Models designed for other diseases have also been validated in the context of the study of patients with OHCA.33 However, we observed that other predictive models have different limitations when applying them in the early assessment of our patients:
- •
In several cases patients not treated with TTM were included in the analyses.24–27
- •
Some models include parameters whose determination is hampered by the sedation required in the first few days.22,24
- •
In other cases, the models use parameters that are not measured at the time of patient admission.24,25
- •
In some studies, the objective was to predict the initial resuscitation outcome (probability of ROSC in the RACA score,23 or probability of death in the first few moments after hospital admission in the NULL-PLEASE score29,31). However, mortality during admission in patients with ROSC continues to be very high (around 50%).
- •
In most of them, the primary outcome was neurological status at discharge,25,26,28 but in the first months after discharge there may still be a certain degree of functional recovery with measures such as rehabilitation, so we believe that it may be more appropriate to consider the situation after the first 6 months have elapsed.
- •
Finally, some models have not been externally validated,24,30,32 which should lead to caution in their application.
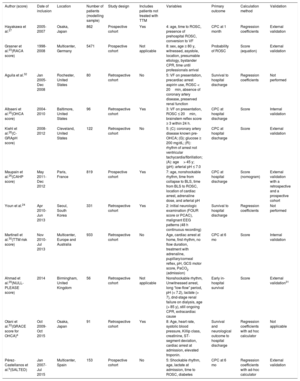
Table 4 shows the characteristics of different models applicable to the prognostic assessment of OHCA patients. Caution should be exercised in the generalization of models that have not undergone external validation. Although the multitude of existing models probably indicates that there is no ideal model, we should also take into account, in addition to their diagnostic power, their ease of use in daily practice. In this regard, we should consider the use of models that can be calculated from a simple score, or with a specifically designed calculator. One of the most robust and widely used models is the CAHP score26; However, an important limitation is that, of the 7 variables required for its application, 3 of them (time from collapse to basic life support, time from basic life support to ROSC, and adrenaline dose) can carry a risk of error given the characteristics of early care for these patients.
Characteristics of the Different Predictive Models Applicable in the Prognostic Assessment of Patients Who Have Had a Cardiac Arrest
| Author (score) | Date of inclusion | Location | Number of patients (modelling sample) | Study design | Includes patients not treated with TTM | Variables | Primary outcome | Calculation method | Validation |
|---|---|---|---|---|---|---|---|---|---|
| Hayakawa et al.27 | 2005-2007 | Osaka, Japan | 862 | Prospective cohort | Yes | 4: age, time to ROSC, presence of prehospital ROSC, conversion to VF | CPC at 1 month | Regression coefficients | External validation |
| Grasner et al.23(RACA score) | 1998-2008 | Multicenter, Germany | 5471 | Prospective cohort | Not applicable | 8: sex, age ≥ 80 y, witnessed, asystole, location, presumable etiology, bystander CPR, time until professionals arrival | Probability of ROSC | Score (equation) | External validation |
| Aguila et al.32 | Jan 2005-Dec 2008 | Rochester, United States | 80 | Retrospective cohort | No | 5: VF on presentation, precardiac arrest aspirin use, ROSC < 20min, absence of coronary artery disease, preserved renal function | Survival to hospital discharge | Regression coefficients | Not performed |
| Albaeni et al.25(OHCA score) | 2004-2010 | Baltimore, United States | 96 | Retrospective cohort | Yes | 3: VF on presentation, ROSC ≤ 20min, brainstem reflex score ≥ 3 within 24 h. | CPC at hospital discharge | Score | Internal validation |
| Kiehl et al.28(C-GRApH score) | 2008-2012 | Cleveland, United States | 122 | Retrospective cohort | No | 5: (C): coronary artery disease known pre-OHCA; (G): glucose ≥ 200 mg/dL; (R): rhythm of arrest not ventricular tachycardia/fibrillation; (A): age> 45 y; (pH): arterial pH ≤ 7.0 | CPC at hospital discharge | Score | External validation |
| Maupain et al.26(CAHP score) | May 2011-Dec 2012 | Paris, France | 819 | Prospective cohort | Yes | 7: age, nonshockable rhythm, time from collapse to BLS, time from BLS to ROSC, location of cardiac arrest, adrenaline dose, and arterial pH | CPC at hospital discharge | Score (nomogram) | External validation with a retrospective and a prospective cohort |
| Youn et al.24 | Apr 2010-Jun 2013 | Seoul, South Korea | 331 | Retrospective cohort | Yes | 2: initial neurologic examination (FOUR score or PCAC), malignant EEG patterns (48 h continuous recording) | Survival to hospital discharge | Regression coefficients | Not performed |
| Martinell et al.30(TTM risk score) | Nov 2010-Jul 2013 | Multicenter, Europe and Australia | 933 | Retrospective cohort | No | Age, cardiac arrest at home, first rhythm, no flow duration, treatment with adrenaline, pupillary/corneal reflex, pH, GCS motor score, PaCO2 (admission) | CPC at 6 mo | Score | Internal validation |
| Ahmad et al.29(NULL-PLEASE score) | 2014 | Birmingham, United Kingdom | 56 | Retrospective cohort | Not applicable | Nonshockable rhythm, Unwitnessed arrest, long “low-flow” period, pH (< 7.2), lactate (> 7), dnd-stage renal failure on dialysis, age (> 85 y), still ongoing CPR, extracardiac cause | Early in-hospital survival | Score | External validation31 |
| Otani et al.33(GRACE score for OHCA)a | Oct 2009-Oct 2015 | Osaka, Japan | 91 | Retrospective cohort | Yes | 8: Age, heart rate, systolic blood pressure, Killip class, creatinine, ST-segment deviation, cardiac arrest at admission, elevated troponin. | Survival and neurological outcome to hospital discharge | Regression coefficients with ad hoc calculator | Not applicable |
| Pérez-Castellanos et al.b(SALTED) | Jan 2007-Jul 2015 | Multicenter, Spain | 153 | Prospective cohort | No | 5: Shockable rhythm, age, lactate at admission, time to ROSC, diabetes | CPC at 6 mo | Regression coefficients with ad-hoc calculator | External validation |
BLS, basic life support; CAHP, Cardiac Arrest Hospital Prognosis; CPC, Pittsburgh Cerebral Performance Category; CPR, cardiopulmonary resuscitation; EEG, electroencephalogram; FOUR, Full Outline of UnResponsiveness; GRACE, Global Registry of Acute Coronary Events; GCS, Glasgow Coma Scale; OHCA, out-of-hospital cardiac arrest; PaCO2, partial pressure of carbon dioxide in arterial blood; PCAC, postcardiac arrest illness severity; RACA, ROSC after cardiac arrest; ROSC, return of spontaneous circulation; TTM, targeted temperature management; VF, ventricular fibrillation.
We believe that our model has several advantages: a) it was performed using patients treated with an intensified postresuscitation approach (including TTM and early coronary angiography when indicated); b) SALTED uses only variables readily available after cardiac arrest; c) this model has been externally validated; and d) a calculator has been designed to facilitate its estimation.
Strengths and LimitationsThe predictive model was obtained with data from 153 patients treated at 2 hospitals and has subsequently been subjected to external validation in a second group of 91 patients admitted to a third hospital. Although there were significant differences between the patients in the 2 groups, the model retained a high diagnostic power.
The usefulness of spectral analysis of fibrillation records in the prognostic assessment of these patients has recently been described.34 This parameter also has the advantage of being available at patient admission, although it was not assessed in our analysis since our study included patients with shockable and nonshockable rhythms.
Probably the most important limitation of this study is the relatively high false-positive rate (12.5%) in a model that aims to determine which patients will have an unfavorable outcome, which means that a few patients may be misclassified by the model as having poor prognosis when their recovery at 6 months might be good. However it is important to emphasize that this estimation should not be taken as a definitive conclusion on the neurological status of the patient, but only as supplementary information to help in initial decision-making and as a support to inform relatives. The study was conducted at 3 large academic centers, and so the results may not be generalizable to other hospitals. Therefore, further studies in different populations are needed to contrast this model. Another limitation is the difficulty of obtaining precise information regarding the time of cardiac arrest, the initiation of resuscitation and its duration, as this information was obtained from prehospital emergency services and witnesses. In survivors of witnessed cardiac arrest (91% of patients in our database), time to ROSC is a good estimation of the duration of circulatory arrest, while in those with unwitnessed cardiac arrest, it may underestimate the true duration of circulatory collapse.
CONCLUSIONSA predictive model based on 5 clinical and easily accessible variables at admission (Shockable rhythm, Age, Lactate levels, Time Elapsed to ROSC, and Diabetes – SALTED) can help to predict the probability of survival without major neurological damage following OHCA.
FUNDINGSupported in partly by the Spanish Ministry of Science and Innovation (Red RIC, PLE2009-0152), the Spanish Health Research Fund (PI14/00857) and Spanish Society of Cardiology (Clinical Research Grants 2015).
CONFLICTS OF INTERESTNone declared.
- –
Unconscious survivors of OHCA have a high risk of death or poor neurologic function.
- –
Neurological damage is frequent and plays a key role limiting the prognosis of these patients.
- –
Although TTM is recommended to improve prognosis, it also limits the role of neurological examination and makes the determination of prognosis in the early moments after resuscitation a difficult task.
- –
The SALTED is a predictive model based on 5 clinical and easily accessible variables at admission (Shockable rhythm, Age, Lactate levels, Time Elapsed to ROSC, and Diabetes) that can provide complementary information to aid in initial decision-making for survivors of OHCA and serve as a support to inform relatives.