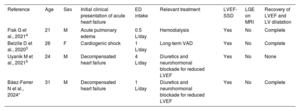
The consumption of energy drinks (EDs) has gradually increased over recent years. In 2011, more than 20 000 visits were made to emergency departments in the United States in relation to EDs, mainly among the younger population.1 These drinks contain between 80 and 160mg of caffeine per can, and caffeine intake >480mg in 24h or >200mg daily has been associated with adverse cardiovascular events.1 Such events include atrial fibrillation, ventricular arrhythmia, ischemic heart disease, myocarditis, cardiopulmonary arrest, and stress cardiomyopathy, among others.1,2 However, the literature contains only 3 reports of nonischemic dilated cardiomyopathy (DCM) related to the use of EDs3–5 (table 1).
Baseline characteristics of patients reported to have dilated cardiomyopathy related to the consumption of energy drinks
| Reference | Age | Sex | Initial clinical presentation of acute heart failure | ED intake | Relevant treatment | LVEF-SSD | LGE on MRI | Recovery of LVEF and LV dilatation |
|---|---|---|---|---|---|---|---|---|
| Fisk G et al., 20214 | 21 | M | Acute pulmonary edema | 0.5 L/day | Hemodialysis | Yes | No | Complete |
| Belzile D et al., 20203 | 26 | F | Cardiogenic shock | 1 L/day | Long-term VAD | Yes | No | Complete |
| Uyanik M et al., 20215 | 24 | M | Decompensated heart failure | 4 L/day | Diuretics and neurohormonal blockade for reduced LVEF | Yes | No | None |
| Báez-Ferrer N et al., 2024* | 31 | M | Decompensated heart failure | 1 L/day | Diuretics and neurohormonal blockade for reduced LVEF | Yes | No | Complete |
EDs, energy drinks; F, female; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; M, male; MRI, magnetic resonance imaging; SSD, severe systolic dysfunction; VAD, ventricular assist device.
We describe a 31-year-old man with no personal or family history of interest who came to the emergency department due to symptoms of left-sided heart failure since 1 week earlier. The previous month, the patient had noticed functional limitation during his usual sports activities but denied symptoms of angina or syncope. On arrival, he presented with a hypertensive emergency in the context of acute pulmonary edema. Electrocardiography showed sinus tachycardia with narrow QRS, but no arrhythmic events during hospitalization. Chest X-ray revealed a congestive pattern, and severe left ventricular dilatation and dysfunction were seen on echocardiography. Blood work only indicated a low glomerular filtration rate of 55mL/min, with serum creatinine of 1.56mg/dL, high-sensitivity troponin T of 56.50 pg/mL during the plateau phase, and an initial N-terminal fragment of pro–brain natriuretic peptide of 1323 pg/mL. The use of opiates, cannabis, cocaine, or other substances of abuse was ruled out.
Once stabilized, the patient was started on the standard quadruple therapy recommended for reduced ejection fraction. Cardiac magnetic resonance imaging showed a left ventricular end-diastolic diameter of 62mm and an ejection fraction of 29%, but no other findings of interest. No myocardial uptake was observed in the gadolinium-enhanced images, and T2-weighted sequences revealed no increase in the intramyocardial signal. Coronary artery disease and an autoimmune or neoplastic etiology were also ruled out. The patient was diagnosed with DCM, and he was referred to the heart failure unit at our hospital.
During outpatient follow-up, neurohormonal treatment for reduced ejection fraction was titrated to the maximum doses. There were no clinical events during follow-up, and all chemistry and cardiac imaging results had returned to normal. Three months after hospital discharge, natriuretic peptides were <50pg/mL and kidney function was also now normal. Cardiac magnetic resonance imaging at 12months showed left ventricular geometry normalization with no development of myocardial fibrosis. Subsequent genetic testing was negative for pathogenic variants linked to DCM. Once the most common causes of DCM were ruled out, a targeted medical history found that the patient had been drinking 4 cans of EDs daily for the past 3 years, but had stopped completely after the clinical episode described. His average intake of EDs accounted for around 320mg of caffeine and 4g of taurine, along with other ingredients, such as ginseng and L-carnitine. The abnormal results described rapidly returned to normal once this cardiotoxic cause was corrected, and any DCM symptoms were ultimately attributed to an excess intake of EDs in recent years once the Naranjo algorithm was applied.6
Cardiotoxicity-induced DCM is most commonly known to be associated with alcohol and chemotherapy. However, to date, EDs have not been considered a possible cause of DCM, even though they contain various ingredients with a synergistic effect likely to destabilize cardiovascular homeostasis.
The cardiovascular effects of caffeine are not well established. However, the hemodynamic effects and clinical events related to excess caffeine intake have been described.1 A daily caffeine intake above 250mg can increase plasma catecholamine levels and lead to greater chronotropism, inotropism, and left ventricular afterload by elevating systemic vascular resistances, due to increased intracellular cyclic adenosine monophosphate.2 Taurine is also present in EDs, and a positive inotropic effect has been reported. Guarana-containing EDs can heighten the effects described because this substance contains xanthine alkaloids which have even higher amounts of caffeine than coffee.5 Stress cardiomyopathy has been related to high plasma catecholamine concentrations, whether sudden or chronic, and may have the same pathophysiologic basis as DCM associated with excessive ED consumption, but still requires research.4
We report on the first case of DCM in Spain possibly associated with the ED consumption and describe the clinical characteristics of previously published episodes. In view of this patient profile, our letter seeks to prevent ED-related cardiovascular events, particularly among the younger population, known to be more likely to consume EDs and experience this kind of cardiovascular event. We also recommend that a medical history aimed at detecting substance abuse in a young patient with DCM also consider EDs as a potential underlying cause. The toxic threshold and the various clinical implications of each ingredient remain to be determined, and the individual or genetic susceptibility should be assessed in each subject.
FUNDINGNone.
ETHICAL CONSIDERATIONSThe study was approved by the Ethics Committee of the Hospital Universitario de Canarias. The patient gave informed consent for publication of his clinical case. The manuscript has been published taking into account possible sex and gender variables in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEThis study was performed without the use of artificial intelligence tools.
AUTHORS’ CONTRIBUTIONSN. Báez-Ferrer: design and planning of the study, follow-up of the clinical case, and writing of the manuscript. P.C. Parra-Esquivel and C.M. Rodríguez-Cabrera: clinical care of the patient in the emergency department. A. Domínguez-Rodríguez: correction of the manuscript. G. Burillo-Putze and P. Avanzas: critical review and correction of the manuscript.
CONFLICTS OF INTERESTP. Avanzas is associate editor of Rev Esp Cardiol; the editorial procedure established by the journal has been followed to ensure impartial management of the manuscript. All other authors state that they have no conflicts of interest.