A recent publication reported an international outbreak of Mycobacterium chimaera infection following heart surgery, through airborne transmission of the bacteria from the heater-cooler units (HCU) used during cardiopulmonary procedures.1 Here, we describe a case of M. chimaera endocarditis on a prosthetic aortic valve.
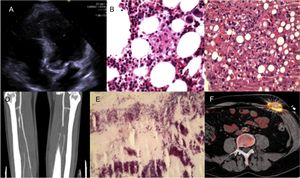
A 51-year-old man was hospitalized in December 2011 for a 3-month history of abdominal pain, fever, hepatomegaly, and weight loss. Six months previously, he had undergone aortic valve replacement with a Mitroflow biologic prosthetic valve due to severe stenosis, with subsequent implantation of a DDD pacemaker to treat postoperative atrioventricular block. The initial analyses showed mild pancytopenia and abnormal liver enzyme levels (aspartate aminotransferase, 412 U/L; alanine aminotransferase, 389 U/L; alkaline phosphatase, 779 U/L; gamma-glutamyl transferase, 460 U/L; total bilirubin, 2.5mg/dL). Transesophageal echocardiography depicted a 17 × 10-mm vegetation anchored in the noncoronary commissure of the aortic valve (Figure A). Amorphous granulomas were seen in liver and bone marrow biopsy specimens, and hematoxylin-eosin staining showed features indicative of hemophagocytosis (Figure B and C). In both specimens, M. chimaera was identified by polymerase chain reaction and culture, which prompted initiation of antibiotic treatment with rifampicin, ethambutol, clarithromycin, and amikacin. The patient required immunosuppressive therapy (etoposide, cyclosporine, and dexamethasone) to control the hemophagocytic syndrome (HPS), which resolved after some weeks of therapy. Two weeks later he experienced occlusion of the tibioperoneal artery due to an embolism (Figure D), in which M. chimaera was again identified (Figure E). In February 2012, the patient underwent Bentall-De Bono surgery to replace the bioprosthesis with a Sorin ART 21 LFA prosthesis, and the endocardial pacemaker with an ESPRIT DR epicardial device. In January 2013, the aortic tube graft with integrated valve had to be replaced with a 21-mm homograft due to dehiscence secondary to uncontrolled infection. The patient experienced an ischemic stroke in August 2013, and a new aortic vegetation was detected. In October 2015, 2 years after the last complication, the medication was withdrawn. The epicardial pacemaker generator was replaced in January 2017 because of battery depletion. The surgical wound from generator replacement showed exudation and lack of healing. Conventional cultures were negative, and finally, M. chimaera was identified by growth in specific media. Antibiotic treatment was reinitiated with azithromycin, rifabutin, and moxifloxacin. Positron emission tomography/computed tomography showed anomalous metabolic activity only at the pacemaker (Figure F). The absence of other metabolic foci in the examination indicated the possibility of complete cure by replacement of the infected system with another endocardial device. At the time of writing, the patient remains in treatment.
Prosthetic aortic valve endocarditis due to Mycobacterium chimaera complicated with hemophagocytic syndrome. A: aortic vegetation at the noncoronary sigmoid commissure. B: hemophagocytosis observed on hematoxylin-eosin staining of bone marrow biopsy. C: hepatic granulomas in biopsy material, made visible by hematoxylin-eosin. D: occlusion of the tibioperoneal artery, seen on angiography. E: acid-alcohol resistant bacilli on hematoxylin-eosin staining of the tibioperoneal embolism material. F: positron emission tomography image.
In 2015, another case of M. chimaera endocarditis was diagnosed in the center where the patient had undergone his first surgery. Samples were taken from the HCU (LivaNova PLC, formerly Sorin Group Deutschland GmbH) and from the hospital environment. The study, performed by reverse hybridization with GenoType NTM-DR (Hain Lifescience Spain S.L.) identified M. chimaera in water samples from the HCU and a tap located outside the surgery rooms. Molecular studies (RAPD-RFLP with IS986 and ERIC PCR) demonstrated a phylogenetic relationship between the strains from both cases.
The case presented here is unusual for 3 reasons. First, it had an atypical presentation with HPS, as few cases of infection caused by slow-growing mycobacteria have been reported to date.2 Second, it was difficult to simultaneously treat the infection and the HPS. Although the infection was treated according to the antibiogram, this technique has not been standardized for atypical mycobacteria, and immunosuppressive treatment has a negative effect on controlling the infection. Finally, this is the first case diagnosed in Spain belonging to an international outbreak of M. chimaera infection following heart surgery. In the last 2 years, cases have been described in many other countries, and new diagnoses are expected.1,3 The use of contaminated HCUs during valve replacement surgery is considered to be the possible source of the infection. The high correlation of M. chimaera DNA obtained from the patients, from the HCH of different countries, and from environmental samples at the manufacture site4 support the hypothesis of contamination of Sorin HCUs at their origin.5 The additional risk of environmental contamination cannot be excluded. The lengthy latency period of these infections points to M. chimaera as a persistent cause of worldwide concern.