Reports of subcutaneous (SC) furosemide used to treat heart failure (HF) are scarce and mainly involve small case series in which this administration route was used for decompensation with congestive symptoms during relatively short periods.1–3 A recently published phase-II clinical trial4 compared the efficacy of intravenous furosemide vs a new SC furosemide formulation for treating decompensated HF, and concluded that the new formulation is similar to the intravenous drug in terms of efficacy and safety. These results suggest that the new formulation may also be effective as long-term treatment for patients with chronic resistance to oral diuretics, who have few therapeutic options.
The main aim of this study was to determine the mid- and long-term effectiveness of HF treatment using SC furosemide infusion with elastomeric pumps, by comparing the hospitalization rates for HF during the year prior to inclusion and during follow-up. The secondary aims were to evaluate the weight reduction in patients with congestive symptoms, assess dry weight maintenance in euvolemic patients starting this therapy, and determine the safety of the intervention. Between December 2014 and March 2018, we recruited 16 consecutive patients with decompensated HF refractory to oral diuretic control and with at least 2 hospitalizations in the previous 6 months or requiring repeat intravenous administration. Severe adverse events were defined as infusion-related infections, local skin lesions leading to treatment discontinuation, and deterioration of renal function or hyperpotassemia requiring treatment. Local skin lesions not requiring specific treatment were considered mild adverse events.
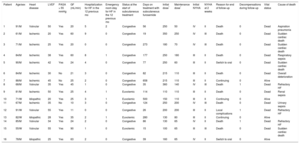
Patient characteristics are shown in Table 1. In the year prior to starting SC furosemide, patients had a mean of a 3.2 ± 2.5 hospitalizations for HF, yielding a rate of 0.26 hospitalizations for decompensated HF/patient/mo of follow-up. All patients had been receiving high doses of oral furosemide (mean dose, 138.7 ± 41.1mg/d; 9 patients [56.3%] took potassium-sparing diuretics and 7 [50.0%] thiazides). Treatment was started during a decompensation or while congestive symptoms persisted in 12 patients. Four patients started treatment while they were euvolemic, after achieving clinical stability with intravenous diuretics.
Characteristics of Patients and Subcutaneous Furosemide Treatment
| Patient | Age/sex | Heart disease | LVEF | PASA > 55 mmHg | GF (mL/min) | Hospitalization for HF in the 12 previous mo | Emergency room stay for HF in the 12 previous mo | Status at the start of subcutaneous treatment | Days on treatment with subcutaneous furosemide | Initial dose* | Maintenance dose* | Initial NYHA | NYHA at 2 weeks | Reason for end of follow-up | Decompensations during follow-up | Vital status | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 91/M | Valvular | 50 | Yes | 20 | 5 | 2 | Congestive | 56 | 250 | 50 | IV | II | Death | 0 | Dead | Aspiration pneumonia |
| 2 | 61/M | Ischemic | 20 | Yes | 60 | 9 | 0 | Congestive | 19 | 350 | 250 | IV | III | Death | 0 | Dead | Sudden cardiac death |
| 3 | 71/M | Ischemic | 25 | Yes | 20 | 0 | 0 | Congestive | 273 | 180 | 70 | IV | III | Death | 0 | Dead | Sudden cardiac death |
| 4 | 84/M | Ischemic | 38 | Yes | 60 | 8 | 1 | Congestive | 177 | 250 | 180 | III | II | Death | 0 | Dead | Respiratory sepsis |
| 5 | 90/M | Ischemic | 42 | Yes | 24 | 4 | 0 | Congestive | 77 | 250 | 60 | III | II | Switch to oral | 0 | Dead | Sudden cardiac death |
| 6 | 84/M | Ischemic | 30 | No | 21 | 3 | 0 | Congestive | 82 | 215 | 110 | III | II | Death | 0 | Dead | Overall deterioration |
| 7 | 88/M | Ischemic | 45 | No | 25 | 2 | 0 | Congestive | 658 | 215 | 110 | III | II | Continuing | 0 | Alive | |
| 8 | 68/M | Valvular | 35 | Yes | 45 | 1 | 0 | Congestive | 35 | 360 | 140 | IV | III | Death | 1 | Dead | Refractory HF |
| 9 | 81/M | Ischemic | 50 | Yes | 25 | 4 | 1 | Euvolemic | 114 | 110 | 110 | III | II | Death | 0 | Dead | Renal sepsis |
| 10 | 71/W | Idiopathic | 20 | Yes | 25 | 3 | 1 | Euvolemic | 500 | 150 | 110 | III | II | Continuing | 0 | Alive | |
| 11 | 67/M | Ischemic | 35 | No | 10 | 3 | 0 | Congestive | 124 | 250 | 200 | IV | III | Death | 0 | Dead | Urinary sepsis |
| 12 | 91/W | Valvular | 55 | Yes | 11 | 0 | 0 | Congestive | 26 | 200 | 200 | III | II | Local complications | 1 | Dead | Refractory HF |
| 13 | 82/W | Idiopathic | 28 | Yes | 35 | 2 | 1 | Euvolemic | 280 | 130 | 80 | III | II | Continuing | 0 | Alive | |
| 14 | 85/M | Valvular | 34 | Yes | 24 | 2 | 0 | Congestive | 86 | 130 | 65 | IV | II | Death | 1 | Dead | Refractory HF |
| 15 | 55/W | Valvular | 55 | Yes | 90 | 1 | 0 | Euvolemic | 15 | 100 | 65 | III | III | Death | 0 | Dead | Sudden cardiac death |
| 16 | 78/M | Idiopathic | 25 | Yes | 63 | 2 | 0 | Congestive | 39 | 160 | 65 | IV | II | Switch to oral | 0 | Alive |
GF, glomerular filtration; HF, heart failure; LVEF, left ventricular ejection fraction; M, man; NYHA, New York Heart Association functional class; PASP, pulmonary artery systolic pressure; W, woman
Two patients did not have hospital admissions, but they experienced decompensation that required intravenous furosemide administration.
The mean duration of therapy with SC furosemide infusion was 159.6 ± 185.1 days. The initial furosemide dose was 234.2 ± 68.4mg/d in congestive patients and 122.5 ± 15.0mg/d in euvolemic patients. During follow-up, only 2 patients experienced a decompensation, with a predominance of signs of low cardiac output; both died during hospital admission. This implied a rate of 0.02 admissions/patient/mo of follow-up. In the remaining patients, there were no decompensations requiring intravenous diuretic administration or hospital admission.
Patients starting SC furosemide therapy with symptoms of congestion experienced weight loss of 2.6 ± 1.0kg in the first 3 days and 0.4 ± 0.3kg in the following days; this loss persisted at 30 days (Figure 1). Those starting SC furosemide after the congestive symptoms had been controlled showed only a slight weight loss.
In 2 patients, SC therapy could be discontinued at 39 and 77 days after initiation, with patients continuing on oral diuretics.
With regard to safety, 2 patients developed local complications (skin erosion without infection) that required treatment discontinuation, permanently in 1 patient and temporarily in the other. There were only 2 infectious complications, which resolved with antibiotics and did not require treatment withdrawal. No significant deterioration of renal function occurred, although there were some mild, transient creatinine increases.
In the group studied (patients attended in a heart failure unit with persistent decompensation attributed to ineffective oral diuretic therapy and requiring intermittent intravenous therapy), SC furosemide administration using elastomeric pumps was useful for decreasing the number of hospitalizations, improving the congestive symptoms, achieving weight reductions in congestive patients, and maintaining the dry weight in euvolemic patients. Adverse events were local and were related to lengthy periods of administration.
Although the number of patients studied is limited, we believe that these promising observations open a new line of investigation for the treatment of patients with refractory HF.