The management of ventricular arrhythmias is complex and often requires the implantation of an implantable cardioverter-defibrillator (ICD). However, antiarrhythmic agents (AAA) continue to be relevant as a primary indication and as a way to reduce device-based therapies in patients with ICDs. However, patients often present contraindications that restrict AAA therapy to just a few options, such as amiodarone. Dronedarone is an AAA that has been proven effective in the control of atrial arrhythmias and may help patients with recurrent ventricular arrhythmias when other drugs cannot; however, information on its effectiveness in this clinical setting is scarce. We describe the use of dronedarone in 3 patients with ventricular arrhythmias who were unresponsive or intolerant to other AAAs.
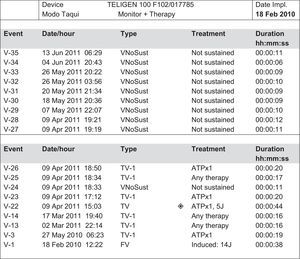
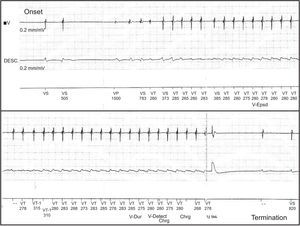
The first patient, a 53-year-old man with hypertension but without structural heart disease, had experienced frequent monomorphic premature ventricular beats since 2007. Treatment with atenolol and sotalol had failed. In February 2010, he was admitted after presenting with syncope and documented a nonsustained monomorphic ventricular tachycardia. An electrophysiology study was conducted and a sustained monomorphic ventricular tachycardia (SMVT) similar to the clinical arrhythmia was induced, as well as 2 other morphologies. Endocardial and epicardial mapping located the arrhythmogenic substrate in the superolateral aspect of the mitral annulus. The application of RF energy was not effective and thus intramyocardial reentry was suspected. Cardiac magnetic resonance imaging showed a scar in the anterolateral aspect of the left ventricle. An ICD was implanted and treatment with flecainide was initiated. Subsequent follow-ups showed that he had experienced numerous episodes of SMVT and received ICD shocks (Figs. 1 and 2). We decided to replace flecainide for dronedarone 400 mg every 12 h and to avoid the use of amiodarone due to its adverse effects. From that time on, and up to his final check-up 14 months later, there was a reduction in arrhythmia burden and the patient did not undergo ICD shocks or experience SMVT episodes, with the exception of 2 episodes that were suppressed by the initial antitachycardia pacing therapy.
The second patient, a 64-year-old man with hypertension but without apparent structural heart disease, had been followed up in another hospital since 2006 for ventricular tachycardia. Three ablation procedures had failed and an ICD was implanted in 2007. Initially, he was treated with metoprolol and subsequently with sotalol and multiple ICD shocks. In 2009, treatment with amiodarone and atenolol was initiated, which reduced the number of episodes. On 2 occasions in December 2011, he was admitted for multiple ICD shocks triggered by SMVT. During his stay he showed nonischemic left ventricle dysfunction (ejection fraction 35%) and thyroid hormone abnormalities (thyrotropin <0.01 μIU/mL; free thyroxine 7.7 ng/dL) associated with the use of amiodarone. During hospital admission he presented a new SMVT episode and received 3 ICD shocks. Amiodarone was not well tolerated and was replaced by dronedarone. After hospital discharge, the patient did not experience ICD shocks during 6 months of follow-up, except for 1 shock that occurred within the first 2 weeks.
The third patient, a 42-year-old man, presented with SMVT in 2005 and was diagnosed with arrhythmogenic right ventricular cardiomyopathy, for which he was treated with sotalol 160 mg/d. In 2012, he was admitted to the emergency department for palpitations and presyncope; SMVT was detected and terminated by electrical cardioversion. During hospitalization, he experienced new SMVT episodes that did not respond to treatment with metoprolol and procainamide. The electrophysiology study showed 3 SMVT morphologies, one of them similar to clinical VT but all of them with poor hemodynamic tolerance that degenerated into ventricular fibrillation. We decided to implant a single-chamber ICD and treat the patient with dronedarone 400 mg every 12 h to avoid the adverse effects associated with amiodarone. During the month following discharge, the patient had 4 SMVT episodes and received multiple shocks. As a result, dronedarone was discontinued and sotalol was restarted, at 160 mg every 12 h; a partial response was achieved.
Dronedarone is a benzofuran derivative that shares the antiarrhythmic properties of amiodarone, but with a better safety profile regarding organ toxicity. It has been proven effective in the treatment of atrial arrhythmias in selected populations.1,2 However, its effectiveness in treating ventricular arrhythmias is less well known. Animal studies have demonstrated its antiarrhythmic effect on ventricular myocardium.3 Its use in humans has been described in 3 isolated cases, with significant reductions reported in arrhythmia burden and the number of ICD shocks.4–6
In this series, which is the largest published to date, a satisfactory response to dronedarone was obtained in 2 patients without structural heart disease; however, in the patient with arrhythmogenic right ventricular cardiomyopathy the decrease in arrhythmia burden was not significant and the drug was discontinued. Furthermore, no adverse clinical or laboratory events were observed and there were no changes in the ICD pacing and sensing parameters. These results, together with previously published findings, support the use of dronedarone in patients with recurrent ventricular arrhythmias in whom other AAAs are considered unsuitable and with no contraindications for its use. However, like other AAAs, its use cannot be expected to be completely effective, especially when other drugs have failed.
CONFLICTS OF INTERESTDr. Merino has acted as a consultant and received financial remuneration for preparing educational presentations on behalf of Sanofi-Aventis.