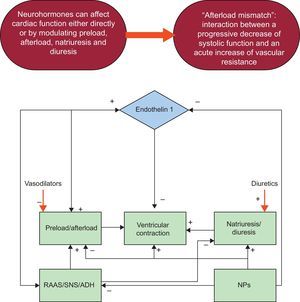
Acute heart failure is globally one of most frequent reasons for hospitalization and still represents a challenge for the choice of the best treatment to improve patient outcome. According to current international guidelines, as soon as patients with acute heart failure arrive at the emergency department, the common therapeutic approach aims to improve their signs and symptoms, correct volume overload, and ameliorate cardiac hemodynamics by increasing vital organ perfusion. Recommended treatment for the early management of acute heart failure is characterized by the use of intravenous diuretics, oxygen, and vasodilators. Although these measures ameliorate the patient's symptoms, they do not favorably impact on short- and long-term mortality. Consequently, there is a pressing need for novel agents in acute heart failure treatment with the result that research in this field is increasing worldwide.
Keywords
Acute heart failure (AHF) is one of the main reasons for hospitalization worldwide. The treatment of chronic heart failure is well defined in the guidelines and has been demonstrated to improve life expectancy in affected patients.1,2 However, for AHF, diuretics, oxygen, and current vasodilators are widely used but have not been shown to reduce mortality. Moreover, there are few large trials on the treatment of AHF in emergency departments (ED) and recommendations in contemporary guidelines are only supported by low levels of evidence.1,2
Nevertheless, it is currently well recognized that the first step in the management of AHF is early management in the ED.3,4
The current approach for patients with AHF in the ED aims to improve the patient's signs and symptoms, correct volume overload, and increase end-organ perfusion and hemodynamic status, conteracting the neurohormonal hyperactivation that is the main physiopathologic mechanism of the disease (Figure 1). It has been demonstrated that an aggressive and appropriate approach to the management of AHF is useful to improve patient outcomes.5,6 Currently, traditional drug therapy is characterized by the use of diuretics, oxygen, and vasodilators, which remains the cornerstone of the early management of AHF.7,8
Despite the earlier initiation of therapy, even when aggressive, mortality in patients with AHF is still very high, demonstrating the need to improve outcomes through the use of new therapeutic strategies.9,10
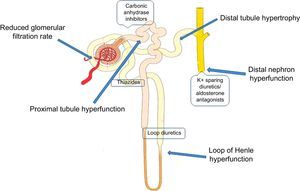
DIURETICSDiuretics still represent the cornerstone of AHF therapy. The current international guidelines consider intravenous (i.v.) loop diuretics as first-line therapy for patients with AHF.2,3,11 This approach aims to improve congestion,12 but evaluation of its efficacy in prolonging survival in AHF has been limited to randomized clinical trials.13 Furosemide, torasemide, and bumetanide are the most commonly used diuretics, and the former is the most widely used in AHF patients. These drugs have different catabolic mechanisms: furosemide is mainly eliminated through the kidney, while torasemide and bumetanide are mainly eliminated through the liver.14 Recent studies by López et al15 in animal models have demonstrated that torasemide, but not furosemide, has an inhibitory effect on aldosterone activity, which leads to decreased fibrotic remodeling in myocytes. Moreover, it has been postulated that torasemide may have a different effect on sympathetic system activation.16
Due to the greater bioavailability of diuretics through intravenous rather than oral administration, i.v. administration is recommended in AHF patients, which allows the diuresis process to start 30 min to 60min after administration. Similar to morphine, i.v. loop diuretics have an initial venodilating effect, which decreases pulmonary congestion before the onset of diuresis.17,18 There is still debate on the dose-response relationship with i.v. diuretics, and often the initial dose is empirical. The guidelines recommend an initial i.v. diuretic dose that equals or exceeds the patient's daily dose in maintenance therapy.11 The ESCAPE trial19 analysis demonstrated that there is a dose-dependent morality risk for i.v. diuretics especially with furosemide that excedes 300 mg/day.19 A single-center study showed that the highest quartile of the daily diuretic dose (> 160mg) has the highest risk-mortality rate.20 Peacock et al21 showed that diuretics could worsen renal function in high doses with consequent poor patient outcomes. However, these data were influenced by different variables (advanced heart failure, renal insufficiency, comorbidities) that increase patient risk for worse outcomes.
In a Cochrane review of 8 clinical trials on AHF of patients randomized to continuous vs bolus loop diuretic administration, those receiving continuous infusion had increased urine output. Because continuous infusion results in a more constant delivery of diuretic to the tubule, it reduces postdiuretic rebound sodium retention and maintains more consistent diuresis.22 However, recently, the double-blind DOSE (Diuretic Optimization Strategies Evaluation), trial randomized patients to low- or high-dose i.v. furosemide, and continuous vs intermittent i.v. furosemide administration. The results showed a trend for greater symptom relief in the high-dose group, with improvement of volume loss and decreased weight at 72hours. In the high-dose group, creatinine levels increased, but this did not influence length of hospital stay or survival. Moreover, it was also demonstrated that the efficacy of continuous infusion was similar to that of intermittent bolus therapy.23
Thiazides are less active diuretics in the management of AHF. They can be used when AHF patients seem to have an inadequate response to loop diuretics, which often occurs in the case of diuretic resistance17 (Figure 2). The combination of metolazone with loop diuretics has been demonstrated to be highly effective.24
The neurohumoral compensatory mechanism in AHF activated by diuretic treatment with stimulation of the sympathetic nervous system and the renin-angiotensin-aldosterone system can act to preserve cardiac output but its prolonged activation may lead to a series of deleterious effects.25 Mineralocorticoid receptor antagonists could help in the treatment of AHF patients not only because they increase diuresis, but also because they attenuate the effects of aldosterone system activation due to loop diuretic use.25,26 Moreover, they attenuate the potassium and magnesium depletion caused by loop diuretics. Nevertheless, spironolactone is a weak diuretic agent when used alone, and can only be used in combination with loop diuretics. In the very early phases of AHF, it should be used at higher i.v. doses than in maintenance therapy. This limits its usefulness because of the presence, in many heart failure patients, of chronic kidney disease, which could be worsened by high doses of mineralocorticoid receptor antagonists.27
VASODILATORSVasodilators, together with diuretics, are the most frequently used drugs in AHF in EDs. These drugs reduce pre- and afterload, or both, by causing arterial and venous dilation, thus lowering left ventricular filling pressure, increasing stroke volume, and improving peripheral oxygen delivery.17 A recent analysis from the ADHERE registry12 demonstrated that patients who received vasoactive agents within 6hours of hospital admission had a significantly lower in-hospital mortality rate and shorter length of stay.17 In a study conducted in patients admitted to the intensive care unit due to AHF, high-dose nitroglycerin (NTG) produces prompt symptom resolution with decreased pulmonary congestion, and a reduced need for mechanical ventilation.28 International guidelines recommend vasodilators in AHF patients as an adjunct to diuretic therapy for rapid resolution of congestive symptoms in normotensive or hypertensive patients, and without severe obstructive valvular disease.2,3,11
The vasodilators most commonly used in AHF management are NTG, nitroprusside, and nesiritide.
Nitroglycerin is an organic nitrate that exerts its function by nitric-oxide mediated smooth muscle vasodilation. It decreases cardiac filling pressures and increases cardiac output. It is mostly used in patients with elevated or normal blood pressure and those with no response or partial response to diuretics. It is preferably used in patients with acute coronary syndromes. Its effect is short-acting and rapid. The initial dosage should be 10-20μg/min and it is increased in 10 to 20 μg increments until the patient's symptoms improve.17,29 A dose of 200μg/min should not be exceeded. A study in which AHF patients were treated with NTG i.v. alone or in combination with furosemide showed that those treated with both drugs had significantly shorter hospital stay,30 a decrease in natriuretic peptides levels, and longer 36-month survival than those treated with either drug alone.
In the VMAC trial,31 patients receiving i.v. NTG showed improved pulmonary capillary wedge pressure at 3hours after the start of treatment. In a small study comparing morphine plus furosemide vs NTG plus acetylcysteine, only small differences were found between treatment groups and there was no advantages of one group over the other.32
Nitroprusside is a potent direct, rapid vasodilator similar in mechanism to NTG. Slow discontinuation is required because of its potential rebound vasoconstriction.29 This drug is started at 10μg/min and is increased to 10-20μg every 10 min to 20 min. Its half-life is very short, 2min, which allows its early instauration in emergency settings.17 Clinical trials evaluating the efficacy of nitroprusside are limited. One retrospective study showed a substantial improvement in cardiac index, lower all-cause mortality, and fewer adverse effects in long-term follow-up compared with patients not receiving nitroprusside.33 A single-center study evaluating nitroprusside in patients with severe aortic stenosis and left ventricular dysfunction showed that the drug rapidly and significantly improved cardiac index at 6 hours and 24hours.34 The main concern in using nitroprusside is its catabolite toxicity, especially in patients with renal and liver failure when a dosage of >3μg/kg/min is used for more than 72hours.35
Nesiritide is the recombinant form of B-type natriuretic peptide. It increases arterial and venous dilation by increasing the intracellular concentration of cyclic guanosine monophosphate (cGMP). It is given at loading dose of 2μg/kg, and then an infusion of 0.01 μg/kg/min to 0.03μg/kg/min for 72hours. In the main trials, the mean duration is 24hours.31 The main mechanism of action is the decrease of left ventricular filling pressure. This drug has been shown to reduce pulmonary capillary wedge pressure and pulmonary vascular resistance and to significantly improve cardiac output compared with NTG and placebo.31 It has a longer half-life than other vasodilators and consequently hypotension, as an adverse effect, can persist for longer. Concern has been raised about the adverse effects of the drug on renal function and on the increased short-term mortality;36,37 for this reason, there has been a decline in the use of this drug.38 A recent meta-analysis tried to counterbalance the results of these publications and showed that first, there was no dose-dependent increase of creatinine in response to the drug at normal dose ranges and, second, that there was a trend in increased mortality at 30 days but not at 180 days. The authors suggested that nesiritide should not be used as a replacement for diuretics or to enhance their action.39 A large recent study (ASCEND-HF [Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure]) that combined nesiritide or placebo with standard therapy reported an improvement in dyspnea in the nesiritide group but also a significant risk for symptomatic and asymptomatic hypotension. However, interestingly, there were no differences in mortality or renal dysfunction in the 2 groups.40 In a multicenter randomized controlled trial, Peacock et al41 showed that there was no significant difference in the hospital admission rate when nesiritide was added to standard therapy in AHF management.
MORPHINEThe use of morphine in AHF management is uncertain. Morphine has been reported to reduce preload and heart rate and has sedative properties. Its net effect is a reduction in myocardial oxygen demand.29 While the European Society of Cardiology guidelines support the use of opiates in AHF management, the American Heart Association guidelines do not.1,2,11 In particular, morphine should be used in the case of acute pulmonary edema. A recent observational analysis of the ADHERE registry suggested that the use of morphine was associated with worse outcomes, including the need for mechanical ventilation, longer length of stay, higher intensive care unit admissions, and higher overall risk-adjusted mortality.42 The Heart Failure Society of America guidelines do not provide a formal recommendation for morphine but state that it should be used with caution.1 A new prospective randomized trial should be performed to evaluate the usefulness or lack of usefulness of morphine in AHF.
OXYGENOxygen is often one of the first-line therapies in AHF together with diuretics and vasodilators. The amount of oxygen is evaluated and administered on the basis of oxygen saturation and arterial blood gas analysis. It is considered a key triage data point.43 Ventilator mechanical support should be considered in AHF patients, in some selected cases, by continuous positive airway pressure support or bilevel positive airway pressure. This could diminish the need for intubation, shorten intensive care unit stay, and decrease mortality.44,45 Noninvasive ventilation is widely used but the effect of this approach on mortality is not well established.
DIGOXINDigoxin could aid the management of AHF patients even though it is not considered a first-line therapy. This drug has vagomimetic effects and decreases the renin-angiotensin system, reduces systemic venous resistance, and increases cardiac output. While European Society of Cardiology guidelines consider the use of digoxin in AHF to control heart rate,2 particularly in the presence of acute atrial fibrillation, the American Heart Association and Heart Failure Society of America guidelines do not indicate its use.1,11 Studies examining clinical outcomes have yet to be conducted on the favorable therapeutic effect of digoxin in AHF.46
ANGIOTENSIN-CONVERTING-ENZYME INHIBITORSWhile angiotensin-converting-enzyme (ACE) inhibitors are widely used in chronic heart failure, their i.v. use in AHF has been little studied. This is due mainly to its adverse effects, such as hypotension, renal dysfunction, and electrolyte imbalance. Therefore, ACE inhibitors do not have a precise role in AHF.29 A small double-blind randomized trial tested i.v. enalaprilat in acute pulmonary edema vs placebo. Enalaprilat showed a greater reduction of pulmonary capillary wedge pressure and improved renal blood flow, but was administered between 6 hours and 18hours after the patients’ arrival at the ED, which prevents these data from being useful in the emergency setting.47 Another study on the efficacy of enalaprilat in patients with hypertensive crises in the ED evaluated its effect in a small cohort of AHF patients and demonstrated that there were no severe adverse effects and the drug aided AHF management.48 However, these 2 studies do not encourage the use of ACE inhibitors in AHF and international guidelines do not endorse their early use.1,2,11
INOTROPESInotropes are used in severe AHF with hypotension and poor cardiac performance.17 Data from the ADHERE registry indicated that 14% of AHF patients in that registry were treated with inotropic agents and these patients had a higher mortality rate (19%) than all other patients. Another study of exacerbations in chronic heart failure showed that milrinone therapy was associated with a significantly higher incidence of hypotension and atrial arrhythmias (OPTIME trial).6,49 The ALARM-HF registry50 reported that the in-hospital mortality rate was much higher in patients receiving i.v. inotropes (25.9%) than in those not receiving these drugs (5.2%) (P < .0001). In view of these findings, current guidelines emphasize the use of inotropes for symptom relief and end-organ dysfunction improvement in patients with low systolic blood pressure (< 90mmHg) and evidence of low output.1,2,11 Some patients could benefit from inotropic agents as a bridge therapy to cardiac transplantation, ventricular assist device, or revascularization.29 The available inotropic agents are dopamine, dobutamine (both beta-adrenergic agonists), and milrinone (type III phosphodiesterase inhibitor). Dobutamine has favorable inotropic and vasodilatory effects with fewer adverse effects on renal dysfunction (hypotension) than milrinone, making it the preferred agent for the management patients with AHF with low output syndrome. It is administered at a starting dose of 1-2μg/kg/min and adjusted on clinical response. Doses > 10μg/kg/min can be associated with an increased incidence of arrhythmias.51
Milrinone can be considered as maintenance therapy together with the use of beta-blockers as they reduce the arrhythmogenic effects of milrinone,52 and as first-line therapy, in preference to dobutamine, in cases of AHF and severe pulmonary hypertension due to its more pronounced vasodilatory properties.53
LEVOSIMENDANLevosimendan is a new inotropic agent calcium sensitizer that enhances myocardial contractility by increasing the affinity of troponin C for calcium without increasing intracellular calcium concentrations.17 It does not cause vasoconstriction because it does not increase epinephrine or norepinephrine concentrations and it ameliorates cardiac output and stroke volume.54 This drug is administered at an initial dose of 6 μg/kg/min to 12μg/kg over 10min and i.v. infusion of 0.1μg/kg/min for 24hours. It has been extensively studied.55–57 The LIDO study55 showed the superiority of levosimendan over dobutamine in reducing capillary wedge pressure and death at 6 months in AHF patients. However, in the SURVIVE trial,56 there were no differences in mortality at 180 days between the group of AHF patients treated with dobutamine and the group treated with levosimendan. A recent trial demonstrated that levosimendan showed no superiority over dobutamine with a trend in favor of levosimendan at 24hours from the start of infusion.57 Two meta-analyses showed that levosimendan has a benefit in terms of long-term survival compared with dobutamine.58,59 However, the results of the various studies on levosimendan vary widely. In a recent secondary analysis of the REVIVE trial,60 levosimendan improved AHF symptoms at the expense of an increase in adverse events such as arrhythmia and hypotension. According to European Society of Cardiology guidelines, levosimendan is considered for AHF patients without severe hypotension when beta-blockade is thought to contribute to hypoperfusion (class B, level C).2
EMERGING MEDICAL THERAPIES FOR ACUTE HEART FAILUREVasopressin Receptor AntagonistsThese new drugs counter the effects of arginine vasopressin, which is increased in heart failure and leads to fluid retention.51 The antagonism of these receptors to the systemic vasculature leads to vasodilation and to free water clearance in the kidney.29 The currently known vasopressin receptor antagonists are conivaptan and tolvaptan. Tolvaptan is indicated for AHF management. A large trial on the efficacy of tolvaptan is the EVEREST study,61 but no differences were found in terms of mortality (both cardiovascular and all-case) or hospitalizations. When tolvaptan was added to traditional therapies, it modestly improved hemodynamics, dyspnea, body weight, and hyponatremia. Conivaptan seems to behave similarly to tolvaptan in AHF patients.62 Both drugs are approved by the Food and Drug Administration for the treatment of hyponatremia. However, currently the international guidelines provide no recommendations on the use of these drugs in AHF.1,2,11
UlaritideUlaritide is a natriuretic peptide, studied in various trials,63 which increases natriuresis and diuresis binding to atrial natriuretic peptide receptors in the kidney. This drug improves hemodynamics and signs/symptoms of AHF apparently without worsening renal function but not infrequently producing severe hypotension.63,64 The TRUE-AHF (TRial of Ularitide's Efficacy and safety in patients with Acute Heart Failure) trial is an ongoing phase III randomized clinical trial designed to evaluate the role of ularitide as an i.v. infusion in addition to conventional therapy in AHF patients. The primary endpoint will be a hierarchic clinical composite variable including a patient-centered assessment of clinical progress, an assessment of lack of improvement or worsening of AHF requiring a prespecified intervention, and death.
RelaxinThe recombinant analog of the endogenous human hormone, relaxin, is currently under study for AHF. Relaxin is a peptide that plays a central role in regulating hemodynamic and renovascular changes during pregnancy.17 It releases nitric oxide, inhibits endothelin and angiotensin II, and produces endothelial growth factor and matrix metalloproteinases.65 Recently, there has been growing interest for this molecule in AHF management. An initial pilot study showed that relaxin has favorable effects in patients with heart failure, including a decrease of ventricular filling pressure and an increase in cardiac output.66 A large placebo-controlled study (Pre-RELAX-AHF)67 showed that relaxin was associated with relief of dyspnea and with a decrease in the combined endpoint of cardiovascular death and readmission at 60 days. In a recent continuation of this study, the RELAX-AHF trial,68,59 patients randomized for relaxin therapy were found to have a significantly greater decrease in dyspnea by visual analog scale over 5 days. Moreover, clinical signs of congestion improved more rapidly and the total dose of i.v. diuretics was significantly lower. The length of hospital stay and coronary care unit stay were significantly decreased in the relaxin group. Patients were enrolled sooner than in other clinical trials within the ED setting.68 Metra et al69 suggested that serelaxin may also have beneficial effects on markers of end-organ damage; in fact, cardiac, renal, and liver values were ameliorated in patients treated with serelaxin, with a significant decrease in creatinine, natriuretic peptide, transaminases, and high sensitivity troponins, all of which are prognostic markers for mortality. This improvement in natriuretic peptides, such as that obtained after treatment with serelaxin,69 should be highly considered, since the positive changes in B-type natriuretic peptide after treatment for heart failure is an increasingly important target for confirming the real benefit for future patient outcomes.70 Nevertheless more data are needed to determine the definitive role of relaxin in patients with acute decompensated heart failure.
RolofyllineRolofylline is an adenosine antagonist. It is thought that adenosine helps to reduce the glomerular filtration rate in heart failure. This antagonist induces, on the contrary, diuresis, by inhibiting the adenosine A1 receptor on afferent arterioles of the kidney. On the basis of this potential beneficial role in AHF, rolofylline was evaluated in an early clinical trial and was demonstrated, in addition to furosemide, to increase diuresis and to prevent a decline in creatinine clearance.71 However, in a recent larger trial (PROTECT trial)72, this drug produced no improvement in AHF symptoms compared with placebo and did not reduce cardiovascular mortality or rehospitalization. Because of its correlation to increased neurologic adverse events, its development was discontinued.73
Omecamtiv MecarbilThis molecule is a cardiac myosin activator that increases the transition of the actin-myosin complex and inhibits the nonproductive hydrolysis of adenosine triphosphate (ATP).74 The final effect is an increase in systolic ejection time, stroke volume, and an amelioration in myocardial oxygen consumption.75 Nowadays, the only published study on this new drug has been performed in patients with chronic heart failure, but not in AHF. A placebo-controlled trial showed that omecamtiv mecarbil improved cardiac function in patients with heart failure caused by left ventricular dysfunction.76 These data were sufficient to proceed with a new study in AHF patients (ATOMIC AHF [Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure]) to further evaluate the potential role of omecamtiv mecarbil. Although it did not achieve its primary endpoint of reducing dyspnea, the cohort that received the highest dose of the drug showed greater dyspnea relief than that receiving placebo, and there were also other favorable dose- and concentration-related trends (data not published by Teerlink et al).77
CledivipineClevidipine is a novel short-acting calcium channel blocker that selectively dilates arterioles and has no effect on myocardial contractility.51 It was first evaluated in a secondary analysis of a small study on hypertension treatment in the ED. The drug also reduced blood pressure rapidly in AHF patients without worsening the heart failure.78 Cledivipine was subsequently studied in a randomized open-label study performed in AHF patients with systolic blood pressure over 160mmHg in the ED. Patients treated with clevidipine had significant dyspnea improvement for up to 3hours, and a blood pressure improvement compared with patients treated with standard vasodilators.79 These results encourage the routine use of cledivipine but cautious titration is necessary because of the significantly greater blood pressure overshoot observed in these patients.
IstaroximeIstaroxime is a peptide that stimulates membrane-bound Na-K/ATPase and enhances the activity of sarcoplasmic reticulum Ca/ ATPase type 2a. It increases inotropy, and improves lusitropy without adverse hemodynamic effects.80 In the HORIZON trial,81 a prospective, double-blind, dose-finding study, istaroxime infusion significantly reduced pulmonary wedge pressure, improved diastolic function and cardiac index, and increased systolic blood pressure in AHF patients compared with placebo.80 These findings demonstrate that this prototype of a new drug could have a potential beneficial effect in patients with low-output AHF.81
CinaciguatCinaciguat is a novel vasodilator acting by activation of soluble guanylate cyclase, thus increasing cGMP production in smooth muscle cells.82 Cinaciguat seems to produce greater vasodilation than nitrates in cases of elevated oxidative stress. Preliminary data in AHF patients seem to show beneficial effects of cinaciguat, with a decrease of systolic blood pressure and improvement of cardiac output.82 The COMPOSE program83 developed a set of 3 randomized, double-blind, placebo-controlled, fixed-dose, multicenter, multinational phase IIb trials aimed at defining the potential role of cinaciguat in AHF. The drug was well tolerated in the pilot clinical study, but a phase II study was prematurely terminated because of hypotensive events that exceeded acceptable levels in patients receiving doses ≥ 200μg/h. In the COMPOSE EARLY trial, hypotension developed in 27.9% AHF patients (vs 5.3% in placebo patients) treated with cinaciguat, and serious treatment-emergent adverse events occurred in 16.3% (vs 10.5% in the placebo group). There was no apparent effect on the cardiac index, and no evidence of dyspnea benefit, leading the study investigators to doubt the usefulness of cinaciguat for AHF. Further studies on the potential use of cinaciguat in emergency settings are underway.
CenderitideCenderitide is a chimeric protein created by the fusion of parts of C-natriuretic peptide and D-natriuretic peptide in order to combine their beneficial effects in AHF patients. The former increases renal blood flow and the latter leads to venous dilation with antialdosterone effects.84 Until now, cenderitide has been tested in healthy volunteers and in a small population of patients with congestive heart failure with hypotension. The drug has been studied as a continuous subcutaneous infusion for use in the postacute heart failure hospitalization period. A phase I pharmacokinetic and pharmacodynamics study was completed in 2011, showing dose-dependent effects on blood pressure over 24hours of infusion.85 A phase II trial targeting enrollment to evaluate cardiac remodeling, renal function, rehospitalization, and mortality as endpoints after 90 days of continuous cenderitide therapy via subcutaneous pump in patients after admission for AHF was planned but not initiated.
CXL-1020CXL-1020 is a pure nitroxyl donor that provides direct positive cyclic adenosine monophosphate (cAMP) independent lusitropic and inotropic effects, as well as combined venous and arterial dilation.86 However, no studies have been developed in AHF patients and further evaluation of CXL-1020 is underway.
TRV120027TRV120027 is a beta-arrestin biased angiotensin II type 1 receptor ligand that has recently been developed for potential use in patients with AHF. This drug acts like a conventional angiotensin receptor blocker, inhibiting angiotensin II-mediated vasoconstriction. A phase II double-blind, placebo-controlled, dose-ranging study targeting enrollment of patients hospitalized for AHF began in 2014 (NCT01187836).
CONCLUSIONSIn conclusion, the pharmacological treatment of the AHF is still based on the use of i.v. diuretics alone or in combination with a series of drugs such as vasodilators (levosimendan and nitrates), angiotensin-converting-enzyme inhibitors, oxygen, digoxin, and morphine. Dopamine should be used in patients with AHF complicated by cardiogenic shock. Currently, there is growing evidence for, and interest in the use of “new” drugs that are potent vasodilators with diuretic and natriuretic mechanisms, such as ularitide and relaxin. Moreover, other molecules, acting at various levels on cardiac smooth muscle cells, arterioles or renal blood flow, are under study. The results are encouraging, and probably these drugs will join very soon the traditional therapy for the useful management of AHF.
CONFLICTS OF INTERESTNone declared.