The study investigated echocardiographic findings after 1 month in 22 patients who received a CoreValve prostheses to treat aortic valve stenosis. Particular attention was paid to the evaluation of valvular leaks and the left ventricular wall thickness. Echocardiograms were obtained prior to implantation, at discharge and 1 month later. The patients mean age was 77±4 years. At discharge, 16 patients (76%) had aortic regurgitation: 8 grade I and 8 grade II. At 1 month, only 13 (62%) presented with the condition: 10 grade I and 3 grade II, with 8 patients (38%) demonstrating a reduction of at least one grade (P<.005). The septal thickness decreased (from 14.2±2mm at baseline to 11±2.4mm at 1 month; P<.001), as did the posterior wall thickness (from 10.9±2.4mm at baseline to 8.3±1.2mm at 1 month; P<.001). In our patient series, the frequency and grade of residual aortic regurgitation after implantation of the CoreValve prosthesis decreased within 1 month, and favorable left ventricular remodeling was also observed.
Keywords
Percutaneous placement of aortic prostheses has been found to be a good alternative in patients with severe aortic stenosis and high surgical risk, with initial success rates of 75%–88%.1,2,3,4 However, there are fewer data concerning their durability or their impact on ventricular function.5 Studies involving the Edwards prosthesis have demonstrated a reduction in the transvalvular gradients,3,6 and parameters such as the left ventricular ejection fraction and left ventricular hypertrophy also have an unquestionable role in the course and prognosis of these patients. Finally, the development of valve leaks following implantation will determine the long-term outcome of these prostheses. To date, there are few studies concerning the early remodeling of the left ventricle, as well as the course of periprosthetic leaks following implantation of the CoreValve prosthesis (CvPr).
Our objective is to evaluate, in our initial series of patients, the early echocardiographic findings, focusing mainly on the grade of prosthetic regurgitation and on left ventricular hypertrophy.
Methods Design and DefinitionsThe present report deals with a case series study. As the main outcome variable, we analyzed the existence and degree of prosthetic regurgitation, scoring it from I (mild) to IV (severe). As secondary variables, we assessed the degree of aortic stenosis, the effective aortic valve area, and left ventricular hypertrophy by measuring the interventricular septum and posterior wall.
PatientsBetween April 24 and December 12, 2008, 22 CoreValve percutaneous aortic bioprostheses were implanted in patients with symptomatic severe aortic stenosis. All of the patients gave their informed consent and a CvPr implantation committee supervised their progress.
Inclusion CriteriaAll the patients who had undergone implantation of a CvPr and had a follow-up of at least one month's duration were included in the study.
Transthoracic Echocardiography (TTE) MeasurementsAll the studies were performed with the same echocardiographic unit (Philips iE33). The Teicholz method was used to assess ventricular function. The degree of prosthetic regurgitation was determined by quantifying the depth and width of the jet, according to the recommendations of other authors and the echocardiographic guidelines,7,8 and the location (paravalvular, transvalvular) and number of jets were also recorded. We measured the peak and mean aortic gradients and the aortic area by means of the continuity equation. The measurements of the ventricular diameters, septal thickness, and left ventricle posterior wall were made using M mode in diastole. The average of three cardiac cycles was taken in the presence of sinus rhythm; five cycles were averaged in the case of atrial fibrillation.
Follow-upAll of the patients who survived the procedures underwent TTE at the time of their discharge from the hospital and one month after implantation to assess the same parameters that were evaluated prior to the procedure.
Statistical AnalysisQuantitative data are presented as the mean plus or minus the standard deviation. Qualitative data are expressed as percentages. For the quantitative variables, fitness to normal distribution was studied using the Kolmogorov–Smirnov test. To compare changes in the quantitative variables during follow-up, Student's t test for paired data or the Wilcoxon test was utilized, depending on whether or not the data fit the normal distribution. For the qualitative variables, the Wilcoxon rank test was employed.
A result was considered to be statistically significant when the P value was less than .05.
ResultsThe mean age of the patients was 77 years (range: 69–82 years) and 50% of them were men. Table 1 summarizes the most relevant baseline characteristics of the study population.
Table 1. Baseline Characteristics of the Patients Included.
| Functional class >II | 8 (36.4) |
| Dyspnea | 20 (86) |
| Angina | 10 (45) |
| Syncope | 5 (23) |
| HT | 13 (59) |
| DM | 4 (18.2) |
| Dyslipidemia | 10 (45.5) |
| Chronic lung disease | 4 (18.2) |
| Peripheral arterial disease | 1 (4.5) |
| Previous cardiac surgery | 2 (9.5) |
| Creatinine (mg/dL) | 0.97 (0.28) |
| SPAP (mmHg) | 42.73 (12.12) |
| PCP (mmHg) | 20 (6.2) |
Abbreviations: DM, diabetes mellitus; HT, hypertension; PCP, pulmonary capillary pressure; SD, standard deviation; SPAP, systolic pulmonary artery pressure.
Data express n (%) or mean (standard deviation).
Following implantation, one of the 22 patients died as a consequence of pericardial tamponade secondary to left ventricular rupture. In the remainder, the procedure was successful, although complete atrioventricular block occurred in seven patients: transient in four (19%) and persistent in three (14%), who required permanent pacemakers.
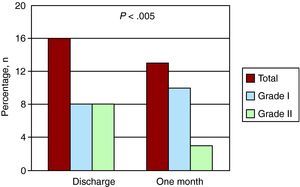
At discharge, 16 of the 21 patients had paravalvular leaks (76% of the population who underwent the follow-up study) (Figure 1). Of these, eight patients (50%) had a grade I leak and the other eight (50%) a grade II leak. In five of the 16 cases (31%), the regurgitation involved two jets at different sites and in the remaining 11 patients there was a single jet. Thus, in all, there were 21 jets at the time of discharge. After one month of follow-up, paravalvular leakage was observed in only 13 patients (62%): grade I in 10 patients (77%) and grade II in three (23%). With respect to the number of regurgitant jets, two different jets remained in only three of the 13 patients (23%) and there was a single jet in the remaining 10, for a total of 16 regurgitation jets after one month of follow-up. There was a significant reduction in the regurgitation, amounting to at least one grade, in eight patients (38%) (P<.005) (Figure 2), as well as a decrease in the absolute number of jets from 21 to 16. Regurgitation did not exceed grade II in any patient and no transvalvular regurgitation was detected.
Figure 1. Numbers of perivalvular leaks at the time of hospital discharge and one month later in patients with Corevalve prostheses.
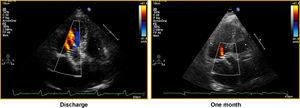
Figure 2. Assessment of prosthetic regurgitation grade prior to hospital discharge and after one month of follow-up in a patient with a Corevalve prosthesis. Discharge: grade II perivalvular leak; one month: grade I perivalvular leak.
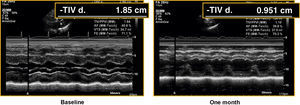
At one month, there was a significant reduction in left ventricle thicknesses and the septum measurement decreased from 14.2±2mm to 11±2.4mm and the posterior wall from 10.9±2.4mm to 8.3±1.2mm (P<.001) (Table 2; Figure 3).
Table 2. Echocardiographic Parameters Prior to the Procedure, at Hospital Discharge and After One Month of Follow-Up in Patients With the CoreValve Prosthesis
| Baseline | Discharge | P | 1 month | P | |
| LVEF, mean (SD), % | 71 (9) | 71.6 (7.6) | NS | 71.9 (5.8) | NS |
| IVS, mean (SD), mm | 14.2 (2) | 13.6 (3) | NS | 11 (2.4) | <.001 |
| PW, mean (SD), mm | 10.9 (2.4) | 10.8 (1.9) | NS | 8.3 (1.2) | <.001 |
| Peak TAoG, mean (SD), mmHg | 93 (17) | 11 (5) | <.001 | 7.4 (4) | <.01 |
| Mean TAoG, mean (SD), mmHg | 60 (12) | 6 (2.5) | <.001 | 7.4 (4) | <.01 |
| AoVA, mean (SD), cm2 | 0.6 (0.2) | 2.4 (0.7) | <.001 | 2.3 (0.9) | <.001 |
| Grade I MI, % of patients | 37 | 38 | NS | 36 | NS |
| Grade II MI, % of patients | 21 | 14 | NS | 19 | NS |
Abbreviations: AoVA, aortic valve area; IVS, interventricular septum; LVEF, left ventricular ejection fraction; MI, mitral insufficiency; NS, not significant; PW, posterior wall; SD, standard deviation; TAoG, transaortic gradient.
All the statistical comparisons are made with respect to the baseline data.
Figure 3. Assessment of left ventricular hypertrophy in a baseline echocardiogram and in an image obtained prior to hospital discharge in a patient with a Corevalve prosthesis. TIVd, interventricular septum thickness.
DiscussionValve regurgitation is a possible technical complication of percutaneous prosthesis implantation. In our study, in contrast to other series,2,5 we had no cases of moderate to severe paravalvular regurgitation (higher than grade II) following implantation.2,5 Moreover, we observed an early reduction in the paravalvular regurgitation grade after CvPr implantation, which was evident after one month of follow-up and probably related to the high adaptability and self-expandability of the nitinol prosthesis. We should also point out the reduction during follow-up in the absolute number of jets, which implies that some of them disappeared completely despite these being the first cases treated by our group, which would include those corresponding to the initial learning curve.
In previous studies, the majority involving Edwards prostheses, mild or moderate paravalvular leaks have been observed after the procedure, with few changes in the degree of severity throughout follow-up. For example, in the series of Webb et al.3 and Cribier et al.,2 with Edwards prostheses, the grade of total prosthetic regurgitation showed no significant changes after one and two years of follow-up, respectively. Likewise, in a series involving the CoreValve prosthesis, Grube et al.1 observed no differences in the prosthetic regurgitation grade over a short-term follow-up. In contrast, Moss et al.5 found that there did appear to be a reduction in the grade of the leaks during the early follow-up period, and the trend persisted for 12 months. However, this reduction proved to be significant only for the transvalvular regurgitations, and is not comparable with our results.
Nevertheless, none of the studies published to date have assessed the course of left ventricular hypertrophy. It can be deduced from our analysis that the hypertrophy, as a mechanism to compensate for the obstruction of left ventricular outflow, is reversible over the short term, a fact that indicates the excellent hemodynamic profile of the prosthesis. In other series, an improvement in left ventricular systolic function has been observed, more marked in patients with baseline dysfunction2,3,5; this was not the case in our study, probably because it did not include patients with ventricular dysfunction.
One limitation to our study is the assessment of the leaks in prosthetic valves, since we have followed the recommendations made by Kapur et al.8 Another limitation is the case series design of the study, with a reduced number of patients and short follow-up period. Studies with the proper design, a larger number of patients and a longer follow-up will establish the definitive role of this therapeutic alternative.
We can conclude that the paravalvular leaks in our series of patients with CoreValve percutaneous aortic prostheses were not severe in any case and that there was an early and significant reduction in their grade and number.
One month after implantation, favorable left ventricle remodeling was already observed, and there was a reduction in ventricular hypertrophy, quite plausibly due to the excellent hemodynamic profile of the prosthesis.
Conflicts of InterestThe authors state that they have no conflicts of interest.
Received 9 July 2009
Accepted 9 February 2010
Corresponding author. Servicio de Cardiología, Hospital Universitario Reina Sofía, Avda. Menéndez Pidal, s/n. 14004 Córdoba, Spain. mamenl@hotmail.com