The recommendations of scientific societies during the COVID-19 pandemic1 have strongly encouraged remote monitoring (RM). There is increasingly widespread use of RM for implantable cardioverter defibrillators (ICDs) as there is evidence that its activation reduces the time to detection of adverse events.2 Nevertheless, its use remains uncommon for pacemaker monitoring, at a rate of 18.5% in Spain in 2020,3 despite having a similar benefit4 and a class IIa indication, or class I for devices on advisory (I C) or patients with limited mobility (I A).5
Large multicenter studies2 support the immediate activation of RM after ICD implantation. However, to our knowledge, this practice is supported by only 1 single-center study after pacemaker implantation.6
We created a multicenter registry (exempt from informed consent, due to its design) with 2 aims: to study the impact of the pandemic on RM of pacemakers and assess the benefit of early (in the first 2 weeks after implantation) activation of pacemaker RM on reducing time to detection of adverse events, using the platform Home Monitoring (HM) from Biotronik (Germany).
The study included 8510 pacemakers from the 5 main manufacturers (table 1), implanted in the year prior to the COVID-19 pandemic lockdown (from 1 March 2019 to 1 March 2020) and the year following the start of lockdown (1 March 2020 to 1 March 2021), in 16 hospitals (10 tertiary and 6 secondary) that were selected, prioritizing high rates of RM and early activation (first 2 weeks). As most of these early activations were performed using the HM platform, the final analysis included only this platform.
Description of the pacemakers and alerts in the FAST REMOTE study
| Pacemakers | Pre-COVID-19 | Post-COVID-19 | |
|---|---|---|---|
| Total | 8510 (100) | 4495 (53) | 4015 (47) |
| RM | 5322 (67) | 2635 (58) | 2687 (67) |
| Early RM | 2979 (35) | 1421 (32) | 1558 (39) |
| Early RM with HM | 1947 (23) | 885 (20) | 1062 (26) |
| Alerts recorded with HM in the first 2 weeks | NS | S |
|---|---|---|
| 880 (97) | 23 (3) | |
| Atrial impedance out of range | 2 | |
| Atrial sensing below limit | 40 | 1 D |
| Atrial autocapture deactivated | 2 | 2 D |
| Ventricular impedance out of range | 14 | |
| Ventricular sensing below limit | 13 | 4 D |
| Ventricular autocapture deactivated | 23 | 2 D, 1 AFa |
| Atrial load over limit | 82 | 4 AFa |
| Long atrial episode | 36 | 1 AFa, 3 AFb |
| Number of atrial episodes over daily limit | 56 | 4 AFa |
| Mode switch counter over limit | 52 | |
| Mode switch duration over limit | 34 | |
| High ventricular rate during mode switches | 6 | |
| Feature of arrhythmia episode | 238 | |
| Percentage ventricular pacing over limit | 74 | |
| Ventricular extrasystole over limit | 47 | 1 D |
| Episodes of high ventricular rate | 52 | |
| Episodes of high ventricular rate over limit | 8 | |
| High mean ventricular rate over limit | 101 |
AFa, unknown atrial fibrillation, alert leading to initiation of OAC (10); AFb, known atrial fibrillation, alert leading to a change in treatment (3); D, lead dysfunction; HM, home monitoring; NS, alert not significant; Pre-COVID-19, period between 1 March 2019 and 1 March 2020 (the year prior to COVID-19 lockdown); Post-COVID-19, period between 1 March 2020 and 1 March 2021 (year since the start of COVID-19 lockdown); RM, remote monitoring; S, alert significant.
Unless otherwise indicated, values are expressed as No. (%).
We recorded a significant increase (9%) in activation of pacemaker RM (58% in the year prior to the COVID-19 lockdown, vs 67% in the year following lockdown; P <.001), but not in early RM (table 1).
Early RM comprised 56% of all RM, equivalent to 2979 pacemakers, of which 1947 (65%) were analyzed using HM. The time from implantation until HM activation was 1.63 ±2 days. In the first 2 weeks of follow-up, 903 alerts were detected in 389 pacemakers (20% of all the pacemakers with HM) (table 1), which were significant in 23 (6% of the pacemakers with alerts) and led to a diagnosis of 10 lead dysfunctions (D) and 13 episodes of atrial fibrillation (AF). The rate of significant alerts (leading to an intervention of some kind) was 1.2% of the pacemakers with HM (0.5% due to dysfunction and 0.6% due to AF).
Time from implantation to lead dysfunction alert detection was 10 (range, 2-14) days and from implantation to AF alert detection, 3 (1-13) days.
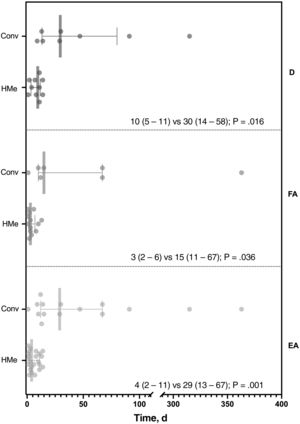
As an indirect measure of the benefit of early RM for the detection of adverse events, we calculated the difference between the median time to detection with HM and the median time to the first scheduled in-office review for the 15 patients with a scheduled appointment (theoretically when the adverse event would have been detected without HM). This difference was statistically significant (figure 1) for lead dysfunction (10 vs 30 days; P=.016), AF episodes (3 vs 15 days; P=.036) and the combination of the 2 (4 vs 29 days; P=.001).
Comparison of time to detection using early home monitoring of lead dysfunction (n=10), episodes of atrial fibrillation (n=13), and the 2 combined (n=23), and time to first in-office scheduled follow-up (n=15). AE, all adverse events; AF, atrial fibrillation; Conv, conventional follow-up; D, lead dysfunction; HMe, early home monitoring.
The FAST REMOTE registry confirms an increase in RM in Spain and, to our knowledge, is the first multicenter study to analyze early RM of pacemakers.
The results confirm the usefulness of early RM after pacemaker implantation, compared with conventional follow-up, for earlier diagnosis of clinically relevant episodes of AF and lead dysfunction, comparable to that seen in studies of ICDs.
The benefit of early RM in terms of reduced time to diagnosis of adverse events may be lesser in asymptomatic patients, but large studies of RM have shown that most detected events are silent; indeed, in our registry, only 1 patient presented with symptoms before being called in as a result of the alert generated.
The main limitations of our study are the selection bias, using centers with a high rate of RM, which may mean a faster response to alerts than in less experienced centers, the inclusion in the analysis of only 1 RM platform, and the retrospective, nonrandomized design. All of these should be borne in mind when interpreting the results, and further studies are warranted.
In conclusion, the FAST REMOTE study shows that RM of conventional pacemakers increased by 9% in the year following the COVID-19 lockdown and that early RM in the first 2 weeks after implantation enabled earlier diagnosis of potentially serious adverse events; therefore, in our opinion it should be implemented as standard, as is done with ICD.
FUNDINGThis study was funded by Biotronik (grant number FF070).
AUTHORS’ CONTRIBUTIONSM. González Vasserot, B. González Chana, C. González Matos, and L. Villagraz Tecedor carried out data collection and analysis. F.J. García Fernández drafted the manuscript. All the authors have reviewed the manuscript and approved the final version.
CONFLICTS OF INTERESTNone.
We thank all the investigators from the FAST REMOTE study (see
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.09.015