The term refractory angina (RA) refers to a clinical picture of chronic angina-like chest pain, lasting for ≥ 3 months and is associated with reversible ischemia that persists despite optimal medical treatment and current percutaneous and surgical revascularization.1 Coronary sinus reducers (CSR) have proven to be effective in reducing symptoms in patients with RA,2 although experience with these devices and available evidence remain scarce.3,4 The aim of the present study was to describe the safety and efficacy of CRSs during an initial experience in Spain.
We conducted an observational retrospective multicenter registry of consecutive patients with RA and CSR implants in Spain. The protocol was approved by a central reference ethics committee, which waived informed consent because the data were guaranteed to be anonymous. The primary efficacy endpoint was change in functional class according to the Canadian Cardiac Society classification (FC-CCS) and the safety endpoint was procedure-related complications.
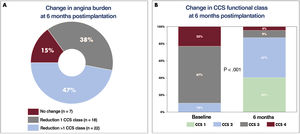
The CSRs were implanted in 48 patients with RA who could not undergo surgical or percutaneous revascularization. Implantation was considered suboptimal in 1 patient because, during follow-up, we observed device shift toward the pulmonary artery, which was asymptomatic (angiographic finding). Table 1 shows the baseline characteristics of the patients, all of whom had documented ischemia in the left coronary territory. One patient died before completing the 6-month follow-up due to causes unrelated to the intervention, and so no follow-up data are available for this patient. At 6 months postimplantation, FC-CCS class improved in 40 patients (85%), and by ≥ 2 classes (P<.001) in 22 (47%) patients (figure 1). The baseline data of the patients show that the severity of angina was higher than that described in previous studies: 90% of our patients were in FC-CCS 3 or 4 before implantation and the patients were taking a mean of 3.8±1.3 antianginal drugs at baseline.
Baseline characteristics of the 48 patients undergoing coronary sinus reducer implantation.
| Clinical characteristics | |
| Age, y | 69±10 |
| Women | 13 (27.1) |
| Hypertension | 43 (89.6) |
| Diabetes mellitus | 25 (52.1) |
| Dyslipidemia | 45 (93.8) |
| Smoking | 5 (10.4) |
| Glomerular filtration rate <60 mL/min/m2 | 17 (35.4) |
| Kidney failure on hemodialysis | 2 (4.2) |
| Previous myocardial infarction | 29 (60.4) |
| Previous PCI | 41 (85.4) |
| Previous coronary intervention | 26 (54.2) |
| Previous stroke or TIA | 5 (10.5) |
| Left ventricular ejection fraction | 53.6±9.9 |
| Drug treatment | |
| Number of drugs | 3.8±1.3 |
| Treatment with beta-blockers | 42 (87.5) |
| Treatment with nondihydropyridine calcium channel blockers | 9 (18.8) |
| Treatment with dihydropyridine calcium channel blockers | 34 (70.8) |
| Treatment with nitrates | 43 (89.6) |
| Treatment with ranolazine | 23 (47.9) |
| Treatment with trimetazidine | 8 (16.7) |
| Treatment with ivabradine | 13 (27.1) |
| Treatment with alopurinol | 7 (14.6) |
| Treatment with antidepressants | 18 (37.5) |
| Angiographic characteristics | |
| Number of vessels with significant stenosis, nonrevascularized | 1.6±1.0 |
| Chronic total occlusion, nonrevascularized | 35 (72.9) |
| Significant disease in common arterial trunk, nonrevascularized | 0 |
| Significant disease in left anterior descending artery, nonrevascularized | 28 (58.3) |
| Significant disease in circumflex artery, nonrevascularized | 29 (60.4) |
| Significant disease in intermediate branch, nonrevascularized | 6 (12.5) |
| Significant disease in right coronary artery, nonrevascularized | 26 (54.2) |
| Significant disease in venous graft, nonrevascularized | 11 (22.9) |
| Significant disease en arterial graft, nonrevascularized | 8 (16.7) |
| Coronary arteries without significant stenosis (microvascular angina disease), nonrevascularized | 5 (10.4) |
TIA, transient ischemic attack; PCI, percutaneous coronary intervention.
Data are expressed as No. (%) or mean±standard deviation.
The greater severity of angina in our patients could explain the responses observed, which were significantly superior to those found in the COSIRA study2 and the RESOURCE and REDUCER-I registries.3,4Figure 1B shows the change in FC-CCS at 6 months postimplantation.
Regarding complications, there was bruising at the puncture site in 2 patients (4.2%), although they did not require transfusion or specific treatment. In 1 patient (2.1%), there was minor coronary sinus dissection, which was documented on angiography and managed without specific treatment. There were no other major implant- or device-related complications during intervention. Regarding incidents, there was device shift in 2 patients during implantation. The devices were recovered without complications through femoral venous access and a second device was successfully implanted in both patients.5
In summary, our initial experience with CSRs in Spain for the treatment of patients with RA has been favorable. Most of the patients experienced symptom improvement without any serious intervention- or device-related complications being reported.
FUNDINGThis work received no external funding for its preparation. World Medica provided funding exclusively for open access publication and was not involved at any point in the process.
AUTHORS’ CONTRIBUTIONSStudy design: O. Rodríguez-Leor and S. Jiménez Valero; manuscript drafting: O. Rodríguez-Leor and S. Jiménez Valero; data collection: all authors; manuscript revision: all authors; statistical analysis: O. Rodríguez-Leor; database review: all authors.
CONFLICTS OF INTERESTO. Rodríguez-Leor has received personal remuneration from World Medica for proctoring cases of coronary sinus reducing device implantation and grants for research projects from Philips Volcano and Shockwave. S. Jiménez Valero has received personal remuneration from World Medica for proctoring cases of coronary sinus reducing device implantation. P. Avanzas is an associate editor of Revista Española de Cardiología; the editorial procedure established by the journal has been followed to ensure impartial handling of the manuscript.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.10.012