This study sought to analyze the association of early coronary angiography with all-cause mortality and cardiovascular mortality in patients with non–ST-segment elevation acute coronary syndrome (NSTEACS) using a large contemporary cohort of patients with NSTEACS from 2 Spanish tertiary hospitals.
MethodsThis retrospective observational study included 5673 consecutive NSTEACS patients from 2 Spanish hospitals between 2005 and 2016. We performed propensity score matching to obtain a well-balanced subset of patients with the same probability of undergoing an early strategy, resulting in 3780 patients. Survival analyses were performed by Cox regression models once proportional risk test were verified.
ResultsAmong the study participants, only 2087 patients (40.9%) underwent early invasive coronary angiography. The median follow-up was 59.0 months [interquartile range, 25.0-80.0 months]. All-cause mortality was 19.0%, cardiovascular mortality was 12.8%, and 51.1% patients experienced at least 1 major cardiovascular adverse event in the follow-up. After propensity score matching, the early strategy was associated with significantly lower mortality (hazard ratio: 0.79; 95% confidence interval 0.62-0.98) in high-risk NSTEACS patients. The darly strategy showed a nonsignificant inverse tendency in patients with GRACE score <140.
ConclusionsIn high-risk (GRACE score≥ 140) NSTEACS patients in a contemporary real-world registry, early coronary angiography (first 24hours after hospital admission) may be associated with reduced all-cause mortality and cardiovascular mortality at long-term follow-up.
Keywords
Abbreviation
Non–ST-segment elevation acute coronary syndrome (NSTEACS) is the most frequent manifestation of acute coronary syndromes.1,2 Coronary angiography plays a central role in this group of patients, allowing confirmation of diagnosis, risk stratification, and the choice of revascularization strategy and antithrombotic therapy.3,4 While routine invasive management is established in high-risk NSTEACS patients, there is still uncertainty regarding the optimal timing of the procedure. In 2 meta-analyses, no differences could be seen with early invasive vs delayed management in terms of outcomes.5,6 However, in the TIMACS study7–that is, those with a high Global Registry of Acute Coronary Events (GRACE) risk score> 140–had a substantial prognostic benefit of an early invasive strategy within the first 24hours. The European Society of Cardiology3 and the American College of Cardiology/American Heart Association guidelines4 recommendation of an early invasive strategy in high-risk patients is based mainly on this subgroup analysis.
Recently, several small randomized clinical trials with low numbers of events have shown that early revascularization reduced mortality; nevertheless, others have failed to demonstrate any significant survival benefit.7–17 In the CRUSADE14 and GRACE15 registries, no apparent benefit of early vs delayed strategies were reported.
The aim of our study was to assess the prognostic impact on mortality of an early invasive strategy in high-risk NSTEACS patients (GRACE score> 140) using a large contemporary cohort of patients with NSTEACS from 2 Spanish tertiary hospitals.
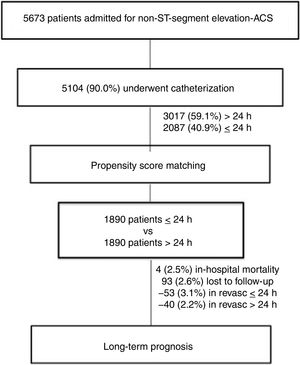
METHODSThe study design included all consecutive patients admitted for NSTEACS in 2 Spanish centers between 2005 and 2016, resulting in a sample cohort of 5673 patients. On-site percutaneous coronary intervention capabilities were available in both centers without the need for transfer. This is a retrospective analysis based on a prospective registry. We analyzed the 5104 (90.0%) patients who underwent angiography during admission. Diagnosis of acute coronary syndrome was established according to current clinical guidelines3,4,18 and patients were classified as having ST-elevation myocardial infarction or NSTEACS according to the electrocardiographic findings. The GRACE score assessed mortality risk, and patients were categorized, according to current recommendations, into 2 groups: low and intermediate (GRACE score between <139) or high risk (GRACE> 140). Early invasive management was defined as one accomplished within the first 24hours of admission, and late invasive intervention was defined as one established after 24hours of hospital admission. After propensity score matching, 1890 patients were assigned to early or late coronary angiography; 2.6% of patients were lost during follow-up (Figure 1). During admission after revascularization of the culprit vessel, patients with multivessel disease underwent complete revascularization guided by an ischemia test, and angina or left ventricular dysfunction.
Risk factors, medical history, treatments, complementary tests and main diagnosis at discharge were collected from all patients by trained medical staff. The diagnostic and therapeutic acute coronary syndrome protocols in both centers include blood sample determinations in the emergency room at arrival and the first fasting state after hospital admission. Glomerular filtration rate was estimated from serum creatinine values with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.19 For the antecedent of previous coronary heart disease, patients needed to have a clinical diagnosis of myocardial infarction, stable or unstable angina, or angina-driven coronary revascularization. Major cardiovascular adverse events (MACE) during follow-up were composed of all-cause mortality, myocardial infarction, hospitalization for heart failure, and unplanned repeat revascularization.
The postdischarge follow-up of patients has a well-established protocol in each center and is composed of telephone calls and review of electronic medical reports and institutional databases. Vital status was ensured by telephone calls in the absence of medical reports. All health-related processes in theses health areas are based on electronic resources in both centers. Patients’ death is always typed in the patients’ electronic database by the primary care physician responsible for out-of-hospital care or by a hospital physician, but the status is changed to “deceased” only by the department of codification of each health area; therefore, vital status is certified by 2 separate processes. Trained medical staff collect and adjudicate clinical events in both databases. The ethics committee of the coordinator hospital approved the study protocol and informed consent was obtained from the patients.
Statistical analysesCategorical variables are expressed as percentages of available data and continuous variables as mean±SD.
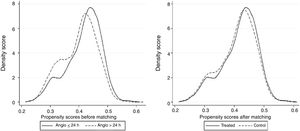
Propensity score matching was performed to obtain a supposedly well-balanced subset of patients with the same probability of early invasive management. This methodology has been largely described, and it equates group characteristics using defined variables to assess the effect of a single variable or treatment. We applied a greedy 1:1 matching algorithm, without replacement, and defined optimal matching as a standard deviation of 0.2. We first performed a binary logistic regression where the dependent variable was “coronary angiography in ≤ 24 hours”, and explanatory variables were age, sex, hypertension, diabetes, dyslipidemia, previous coronary heart disease, heart failure or stroke, GRACE score, and medical treatments at discharge. The density of the propensity score was quite high in the complete population group (Figure 2A), which facilitated the creation of a new subset of well-balanced patients without excluding many patients (Figure 2B). The results were used to determine the covariates in the propensity score matching, which provided a sample of 1890 pairs of patients with the same probability of early strategy. The predictive capacity of the model used to generate the propensity score was 0.72 (95% confidence interval [95%CI] 0.70 - 0.88; P <.01) and exhibited a good fit (Hosmer-Lemeshow P=.80).
The cumulative probability of all-cause mortality, cardiovascular mortality and major cardiovascular adverse events was calculated using the Kaplan-Meier method, while the log-rank test was used to compare the survival distributions of 2 samples. Cox regression models performed survival analyses once the proportional risk tests were verified. Multivariate analyses were adjusted by all variables that obtained P values <.1 in the univariate analysis or if they could have plausible clinical implication; results are presented as hazard ratio (HR) and 95%CI. Harrell's C-statistic was used to test the model's discriminative accuracy and the Gronnesby and Borgan test was used to assess its calibration. Statistical differences were attributed when P <.05. All analyses were performed using STATA 14.3 (StataCorp 2009 Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
RESULTSFrom November 2005 to November 2016, 5673 patients with NSTEACS were admitted, and 5104 (90%) of these underwent catheterization, with available mean follow-up of 59.0 months for mortality outcomes. Of those invasively treated, 3017 (59.1%) patients underwent late invasive coronary angiography, whereas 2087 (40.9%) had early invasive coronary angiography (≤ 24hours). After propensity score matching, 1890 patients were assigned to either an early or a late group according to time of revascularization after admission.
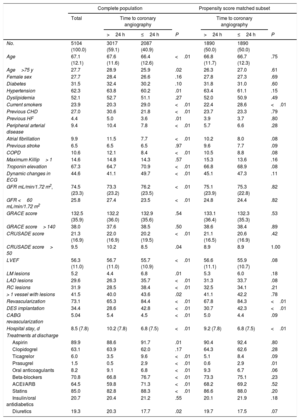
The baseline characteristics of the patients stratified according to time of invasive coronary angiography are described in Table 1. Briefly, the mean age was 67.1 years; 72.3% of the patients were male and the medium GRACE score was 132.5. Patients undergoing the early strategy were younger (66.4±12.6 years vs 66.7±11.6 years) and were more often male (73.4% vs 71.6%). Current smoking was more common (29% vs 20.3%) and glomerular filtration rate was higher (76.2±23.5 vs 73.3±23.2) in the early intervention group, but hypertension (60.2% vs 63.8%) was less prevalent. Previous heart failure, atrial fibrillation, chronic obstructive pulmonary disease and peripheral artery disease were less prevalent in the early intervention group. No differences were seen regarding the mean GRACE score (132.9±35.6 vs 132.2±36.0) between the 2 groups. The length of hospital stay was lower in the early group (6.8 vs 10.2 days); this difference was significantly maintained after the propensity score matching analysis (6.8 vs 9.2 days). After this statistical analysis, the rest of the baseline differences, as well as medical treatment at discharge, disappeared after the propensity analysis except for current smoking, hospital stay, and revascularization rate (84.3% early and 67.8% late invasive strategy) (Table 1). Except for the fact that 259 patients had undergone coronary artery bypass surgery, no differences were shown between the 2 groups.
Patients’ clinical features according to timing of revascularization in the complete cohort and in the propensity score matched subset of patients
| Complete population | Propensity score matched subset | ||||||
|---|---|---|---|---|---|---|---|
| Total | Time to coronary angiography | Time to coronary angiography | |||||
| >24 h | ≤24 h | P | >24 h | ≤24 h | P | ||
| No. | 5104 (100.0) | 3017 (59.1) | 2087 (40.9) | 1890 (50.0) | 1890 (50.0) | ||
| Age | 67.1 (12.1) | 67.6 (11.6) | 66.4 (12.6) | <.01 | 66.8 (11.7) | 66.7 (12.3) | .75 |
| Age>75 y | 27.7 | 28.9 | 25.9 | .02 | 26.3 | 27.0 | .61 |
| Female sex | 27.7 | 28.4 | 26.6 | .16 | 27.8 | 27.3 | .69 |
| Diabetes | 31.5 | 32.4 | 30.2 | .10 | 31.8 | 31.0 | .60 |
| Hypertension | 62.3 | 63.8 | 60.2 | .01 | 63.4 | 61.1 | .15 |
| Dyslipidemia | 52.1 | 52.7 | 51.1 | .27 | 52.0 | 50.9 | .49 |
| Current smokers | 23.9 | 20.3 | 29.0 | <.01 | 22.4 | 28.6 | <.01 |
| Previous CHD | 27.0 | 30.6 | 21.8 | <.01 | 23.7 | 23.3 | .79 |
| Previous HF | 4.4 | 5.0 | 3.6 | .01 | 3.9 | 3.7 | .80 |
| Peripheral arterial disease | 9.4 | 10.4 | 7.8 | <.01 | 5.7 | 6.6 | .28 |
| Atrial fibrillation | 9.9 | 11.5 | 7.7 | <.01 | 10.2 | 8.0 | .08 |
| Previous stroke | 6.5 | 6.5 | 6.5 | .97 | 9.6 | 7.7 | .09 |
| COPD | 10.6 | 12.1 | 8.4 | <.01 | 10.5 | 8.8 | .08 |
| Maximum Killip> 1 | 14.6 | 14.8 | 14.3 | .57 | 15.3 | 13.6 | .16 |
| Troponin elevation | 67.3 | 64.7 | 70.9 | <.01 | 66.8 | 68.9 | .08 |
| Dynamic changes in ECG | 44.6 | 41.1 | 49.7 | <.01 | 45.1 | 47.3 | .11 |
| GFR mL/min/1.72 m2, | 74.5 (23.3) | 73.3 (23.2) | 76.2 (23.5) | <.01 | 75.1 (23.9) | 75.3 (22.8) | .82 |
| GFR <60 mL/min/1.72 m2 | 25.8 | 27.4 | 23.5 | <.01 | 24.8 | 24.4 | .82 |
| GRACE score | 132.5 (35.9) | 132.2 (36.0) | 132.9 (35.6) | .54 | 133.1 (36.4) | 132.3 (35.3) | .53 |
| GRACE score> 140 | 38.0 | 37.6 | 38.5 | .50 | 38.6 | 38.4 | .89 |
| CRUSADE score | 21.3 (16.9) | 22.0 (16.9) | 20.2 (19.5) | <.01 | 21.1 (16.5) | 20.6 (16.9) | .42 |
| CRUSADE score> 50 | 9.5 | 10.2 | 8.5 | .04 | 8.9 | 8.9 | 1.00 |
| LVEF | 56.3 (11.0) | 56.7 (11.0) | 55.7 (10.9) | <.01 | 56.6 (11.1) | 55.9 (10.7) | .08 |
| LM lesions | 5.2 | 4.4 | 6.8 | .01 | 5.3 | 6.0 | .18 |
| LAD lesions | 29.6 | 26.3 | 35.7 | <.01 | 31.3 | 33.7 | .08 |
| RC lesions | 31.9 | 28.5 | 38.4 | <.01 | 32.5 | 34.1 | .21 |
| > 1 vessel with lesions | 41.5 | 40.0 | 43.6 | .02 | 41.1 | 42.2 | .78 |
| Revascularization | 73.1 | 65.3 | 84.4 | <.01 | 67.8 | 84.3 | <.01 |
| DES implantation | 34.4 | 28.6 | 42.8 | <.01 | 30.7 | 42.3 | <.01 |
| CABG revascularization | 5.04 | 5.4 | 4.5 | <.01 | 5.0 | 4.4 | .09 |
| Hospital stay, d | 8.5 (7.8) | 10.2 (7.8) | 6.8 (7.5) | <.01 | 9.2 (7.8) | 6.8 (7.5) | <.01 |
| Treatments at discharge | |||||||
| Aspirin | 89.9 | 88.6 | 91.7 | .01 | 90.4 | 92.4 | .80 |
| Clopidogrel | 63.1 | 63.9 | 62.0 | .17 | 64.3 | 62.6 | .28 |
| Ticagrelor | 6.0 | 3.5 | 9.6 | <.01 | 5.1 | 8.4 | .09 |
| Prasugrel | 1.5 | 0.5 | 2.9 | <.01 | 0.6 | 2.9 | .01 |
| Oral anticoagulants | 8.2 | 9.1 | 6.8 | <.01 | 9.3 | 6.7 | .06 |
| Beta-blockers | 70.8 | 66.8 | 76.7 | <.01 | 73.3 | 75.1 | .23 |
| ACEI/ARB | 64.5 | 59.8 | 71.3 | <.01 | 68.2 | 69.2 | .52 |
| Statins | 85.0 | 82.8 | 88.3 | <.01 | 86.6 | 88.0 | .20 |
| Insulin/oral antidiabetics | 20.7 | 20.4 | 21.2 | .55 | 20.1 | 21.9 | .18 |
| Diuretics | 19.3 | 20.3 | 17.7 | .02 | 19.7 | 17.5 | .07 |
ACEI, angiotensin converting-enzyme inhibitors; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CHD, coronary heart disease; DES, drug-eluting stents; HF, heart failure; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LAD, left anterior descending; LM, left main; LVEF, left ventricle ejection fraction; STEMI, ST-elevation myocardial infarction.
Unless otherwise indicated, the data are presented as No. (%).
A small percentage (10%) of the initial population did not undergo coronary angiography. These patients, who did not undergo catheterization, had significantly higher age (84.0±4.7 vs 81.0±4.7), higher scores on the GRACE scale (174.4±36.1 vs 158.3±32.9) and worse glomerular filtration (57.7±23.1 vs 65.5±29.7).
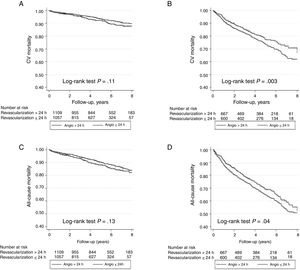
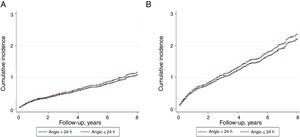
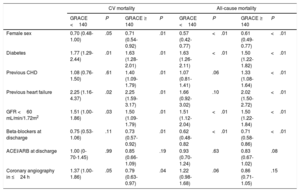
Overall mortality in this population of NSTEACS patients was low. There were 2.5% (94 patients) deaths occurring in-hospital, 1.6% in the early invasive strategy, and 3.1% in the late invasive strategy (P=.001). At 59.0 months, all-cause mortality occurred in 18.8% (699 patients) and 12.5% (471 patients) related to cardiovascular causes. All-cause mortality and cardiovascular mortality were lower in patients undergoing early coronary angiography than in those with late coronary angiography (16.1% vs 21.5%; P <.001 and 10.9% vs 14.1%; P=.002, respectively). As shown in the Kaplan-Meier curves (Figure 3), patients under an early strategy had lower all-cause mortality (log-rank test; P=.04) and cardiovascular mortality (log-rank test; P=.03) in patients with GRACE score> 140; however, these differences were not observed in patients with GRACE score <140 (log-rank test; P=.11 and P=.13 for all-cause mortality and cardiovascular mortality, respectively). After adjustment for confounding variables, the early strategy in NSTEACS high-risk patients compared with the late strategy was associated with a significant reduction in cardiovascular mortality (HR, 0.79; 95% confidence interval, 0.63-0.97); a nonsignificant difference was found regarding all-cause mortality in high-risk patients (HR, 0.86; 95% confidence interval, 0.71-1.05) among the 2 invasive strategies. Nevertheless, in low-intermediate risk patients, no significant differences were found according to time of intervention (Table 2). Heart failure hospitalization was lower in patients undergoing an early strategy (10.2% vs 15.3%, P <.01), but in the multivariate analysis with competing risk, taking death as a competitive event, there was no statistically significant association (subdistribution hazard ratio, 0.89; 95% confidence interval, 0.72-1.12; P=.35). In addition, during follow-up, 12.8% (471 patients) had a first hospital admission for heart failure, with no differences between groups, and 51.1% (1653 patients) experienced at least 1 major cardiovascular adverse event with a greater number of events in the late strategy (group P=.03) (Figure 4).
Results of the multivariate analysis for cardiovascular and all-cause mortality
| CV mortality | All-cause mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| GRACE <140 | P | GRACE ≥ 140 | P | GRACE <140 | P | GRACE ≥ 140 | P | |
| Female sex | 0.70 (0.48-1.00) | .05 | 0.71 (0.54-0.92) | .01 | 0.57 (0.42-0.77) | <.01 | 0.61 (0.49-0.77) | <.01 |
| Diabetes | 1.77 (1.29-2.44) | .01 | 1.63 (1.28-2.01) | .01 | 1.63 (1.26-2.11) | <.01 | 1.50 (1.22-1.82) | <.01 |
| Previous CHD | 1.08 (0.76-1.50) | .61 | 1.40 (1.09-1.79) | .01 | 1.07 (0.81-1.41) | .06 | 1.33 (1.08-1.64) | <.01 |
| Previous heart failure | 2.25 (1.16-4.37) | .02 | 2.25 (1.59-3.17) | .01 | 1.66 (0.92-3.02) | .10 | 2.02 (1.50- 2.72) | <.01 |
| GFR <60 mL/min/1.72m2 | 1.51 (1.00-1.86) | .03 | 1.50 (1.09-1.79) | .01 | 1.51 (1.12-2.04) | <.01 | 1.50 (1.22-1.84) | <.01 |
| Beta-blockers at discharge | 0.75 (0.53-1.06) | .11 | 0.73 (0.57-0.92) | .01 | 0.62 (0.48-0.82 | <.01 | 0.71 (0.58-0.86) | <.01 |
| ACEI/ARB at discharge | 1.00 (0-70-1.45) | .99 | 0.85 (0.66-1.09) | .19 | 0.93 (0.70-1.24) | .63 | 0.83 (0.67-1.02) | .08 |
| Coronary angiography in ≤24 h | 1.37 (1.00-1.86) | .05 | 0.79 (0.63-0.97) | .04 | 1.22 (0.98-1.68) | .06 | 0.86 (0.71-1.05) | .15 |
ACEI, angiotensin converting-enzyme inhibitors; ARB, angiotensin receptor blocker; CHD, coronary heart disease; CV, cardiovascular; GFR, glomerular filtration rate.
Data are presented as hazard ratio (95% confidence interval).
In this contemporary real-world registry of 2 tertiary Spanish centers, early coronary angiography in high-risk NSTEACS patients (GRACE> 140) was associated with lower early and long-term all-cause mortality and cardiovascular mortality compared with a delayed strategy; the higher proportion of patients revascularized in the early invasive group may contribute to explain our findings. To the best of our knowledge, this study describes for the first time in a real-world contemporary registry that an early invasive strategy improves long-term prognosis in high-risk NSTEACS patients. Our results may have several implications in clinical NSTEACS management and in health system organization. First, they add information from a large, contemporary cohort of patients, and reinforce the recommendations of the European Society of Cardiology3 and American College of Cardiology/American Heart Association4 guidelines for management of high-risk NSTEACS patients. Second, our results also suggest that a “network care system” for high-risk NSTEACS patients needs to be developed to allow for an early invasive coronary strategy, particularly for hospitals without on-site 24-hour catheterization facilities, that function at the weekends and during the holiday season. Third, our work supports a longer-term (almost 5-year follow-up period) prognostic benefit of the early invasive strategy compared with previous publications; this effect was observed early and the curves continued to diverge during the entire follow-up. As previously mentioned, the higher revascularization rate in the early invasive group may be involved in these findings.
Several trials have explored the optimal timing of invasive strategies and have yielded conflicting results. Deharo et al.8 have recently described the association of early coronary angiography in NSTEACS patients with a GRACE score> 140. Using the large contemporary cohort of NSTEACS patients extracted from the Treatment of Acute Coronary Syndrome With Otamixaban randomized trial, an early invasive strategy among high-risk patients was associated with a lower risk of death and myocardial infarction at 180 days. With coronary angiography> 24hours as a reference, coronary angiography from 12 to 24hours was not associated with a lower risk of primary ischemia outcomes at 180 days, while coronary angiography <12hours was associated with lower mortality and myocardial infarction risks; performing coronary angiography <12hours was associated with better prognosis compared with the other subgroup (12-24hours). The rate of revascularization was significantly higher in very early and early invasive strategies and may help to explain the better outcomes in these groups. The authors conclude that this important clinical observation deserves confirmation over a longer follow-up period by other contemporary prospective registries and, as previously mentioned, has potential significant implications for the care organization of patients with NSTEACS. Similar results have been described by a Danish trial that included 2147 patients with NSTEACS; early coronary angiography only improves prognosis in high-risk patients (GRACE≥ 140). As previously mentioned, the higher rate of revascularization in the early strategy may influence in these results.20
Our results contribute to knowledge beyond the finding of the TIMACS trial.7 TIMACS did not show differences between an early and a delayed invasive strategy in patients with low and intermediate risk. However, death, myocardial infarction and stroke at 6 months were reduced in patients with a GRACE score> 140 with an early (≤ 24hours) compared with a delayed (> 36hours) invasive strategy. Again, the higher rate of revascularization in the early intervention group may be involved in the higher prognostic benefit in this group. Similar results were seen in the ACUITY trial.9 Based on this evidence, the American4 and European guidelines3 recommend the early invasive strategy as class I in high-risk NSTEACS patients. Our results portray a contemporary real-world registry in the same high-risk NSTEACS patients, with longer follow-up, reinforcing the current guidelines’ recommendations. Two recent meta-analyses and the MINAP registry showed the prognostic impact of the early invasive strategy in NSTEACS patients. The magnitude of the benefit seems to be time- and risk-dependent according to the GRACE risk score.10,21,22
In a real-world analysis of the Atherosclerotic Risk in Communities surveillance study including 16 383 NSTEACS hospitalized patients undergoing coronary revascularization, early percutaneous intervention was associated with a better 28-day survival, both for the entire population and the subgroup of patients classified as high risk. However, by 1-year of follow-up, the better survival with early coronary intervention was no longer statistically significant.11 The Atherosclerotic Risk in Communities study results are consistent with a few previous studies, such as RIDDLE-NSTEMI12 and the ISAR-COOL.13 Nevertheless, different demographic and clinical characteristics among these trials prevent definitive conclusions. In contrast with the results of these trials and registries, in the older registry data from CRUSADE14 and GRACE,15 no apparent survival benefit with an early invasive strategy was observed, even though a higher rate of revascularization was found in the early invasive strategy group in GRACE. The more modern OPTIMA16 and ABOARD17 trials showed no benefit and even possible harm (increase in the incidence of ischemic events in OPTIMA) with immediate revascularization. Accordingly, an immediate invasive approach in NSTEACS, similar to the strategy used in primary percutaneous coronary intervention, has not established benefits and, according to the current guidelines, is only recommended in severe unstable patients. The fact that oral antiplatelet agents need at least 3 to 4hours after a loading dose to be effective may play a possible role in explaining the absence of benefit, including harm, of an immediate invasive strategy in NSTEACS patients. Our results are consistent with the benefit, in terms of early and long-term survival, of a more conservative approach and suggest that coronary strategies in the first 24hours after hospital admission are consistently associated with an improved prognosis in the group of patients with higher risk (GRACE score> 140).
The present study has several limitations. A potential weakness is the retrospective nature of this analysis. This is an observational registry with its inherent limitations (eg, selection bias, differences in groups with regard to baseline characteristics, unmeasured bias), and thus associations between various treatments and outcomes may be confounded by unmeasured variables. Several unmeasured confounders or details about physician or patient decision-making might not be available in our data collection protocol and could account for some of the reported findings. In addition, the use of ticagrelor and prasugrel in the antithrombotic management of patients with NSTEACS has been incorporated in recent years, with a very low percentage of our population treated with these 2 drugs. Finally, long-term outcomes could be modified by many circumstances that might not be available or controlled in the follow-up protocol of our centers. As such, the results reported in this analysis should be considered hypothesis-generating and merit confirmation in other registries and clinical trials. A more homogenous and vast use of optimal medical therapy supported by clinical guideline recommendations in these patients, particularly a greater use of more active antiplatelet drugs, may influence our results.
CONCLUSIONSIn high-risk (GRACE score> 140) NSTEACS patients in a contemporary real-world registry, an early invasive strategy (first 24hours after hospital admission) might be associated with reduced all-cause mortality and cardiovascular mortality after hospital discharge and at long-term follow-up. These results reinforce the current guideline recommendations for an early strategy in NSTEACS patients with a high risk of ischemic events.
CONFLICTS OF INTERESTNone declared.
- -
Coronary angiography plays a central role in non–ST-elevation myocardial infarction patients, allowing confirmation of diagnosis, risk stratification, and the choice of revascularization strategy and antithrombotic therapy. While a routine invasive management is established in high-risk NSTEACS patients, there is still uncertainty regarding the optimal timing of the procedure. Although ESC guidelines recommended an early strategy in high-risk patients, several studies have reported heterogeneous results.
- -
This is the first time that early coronary angiography is evaluated in patients with high-risk NSTEACS in a real-world Spanish registry.
- -
Our results reinforce the recommendations of clinical practice guidelines.
- -
Early coronary angiography in patients with high-risk NSTEACS is associated with a reduction in all-cause mortality and cardiovascular mortality.