Economic studies may help decision making in the management of multivessel disease in the setting of myocardial infarction. We sought to perform an economic evaluation of CROSS-AMI (Complete Revascularization or Stress Echocardiography in Patients With Multivessel Disease and ST-Segment Elevation Acute Myocardial Infarction) randomized clinical trial.
MethodsWe performed a cost minimization analysis for the strategies (complete angiographic revascularization [ComR] and selective stress echocardiography–guided revascularization [SelR]) compared in the CROSS-AMI clinical trial (N=306), attributable the initial hospitalization and readmissions during the first year of follow-up, using current rates for health services provided by our health system.
ResultsThe index hospitalization costs were higher in the ComR group than in SelR arm (19 657.9±6236.8 € vs 14 038.7±4958.5 €; P <.001). There were no differences in the costs of the first year of follow-up rehospitalizations between both groups for (ComR 2423.5±4568.0 vs SelR 2653.9±5709.1; P=.697). Total cost was 22 081.3±7505.6 for the ComR arm and 16 692.6±7669.9 for the SelR group (P <.001).
ConclusionsIn the CROSS-AMI trial, the initial extra economic costs of the ComR versus SelR were not offset by significant savings during follow-up. SelR seems to be more efficient than ComR in patients with ST-segment elevation acute coronary syndrome and multivessel disease treated by emergent angioplasty.
Study registred at ClinicalTrial.gov (Identifier: NCT01179126).
Keywords
Cardiovascular disease is the leading cause of mortality in Western countries, with acute myocardial infarction occupying the top position.1,2 Percutaneous coronary intervention (PCI) is the reperfusion therapy of choice for patients with acute ST–segment-elevation acute myocardial infarction (STEMI).3 Approximately 40% to 60% of patients with STEMI have multivessel coronary artery disease and a worse clinical prognosis.4,5
The current recommendation for treating multivessel disease in patients with STEMI is to consider revascularizing nonculprit lesions, above all to reduce the need for revascularization and the risk of death or infarction following discharge.3,6 The conservative arms in trials conducted so far, however, have been excessively conservative, as the only artery revascularized is the infarct-related artery (IRA), with no consideration given to the potential functional impact of nonculprit lesions. The true benefits of complete revascularization, therefore, may be overestimated.7
While decision-making should be guided by the patient's clinical profile and the safety and effectiveness of proposed interventions, economic evaluations reflecting the cost of various procedures are helpful for complex decision-making scenarios, particularly when resources are limited.8,9
Multivessel disease in patients with STEMI provides an ideal setting for economic evaluations, as treatment is both complex and costly, and several aspects, such as how best to select nonculprit lesions, remain to be clarified before systematic complete revascularization can be recommended.7
The aim of this study was to conduct an economic evaluation nested in the CROSS-AMI (Complete Revascularization or Stress Echocardiography in Patients With Multivessel Disease and ST-Segment Elevation Acute Myocardial Infarction) clinical trial.
MethodsCROSS-AMi trial designAs previously reported,10 the CROSS-AMI trial was a multicenter randomized clinical trial of patients with STEMI and multivessel disease comparing complete anatomic revascularization of all nonculprit coronary lesions during initial hospitalization (ComR) with selective revascularization based on stress echocardiography-guided detection of ischemia (SelR).10 The trial received ethics committee approval and was conducted in accordance with the principles of the Declaration of Helsinki (reference 2010/160). It was registered at ClinicalTrials.gov under identifier number NCT01179126.
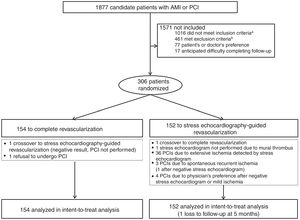
A total of 306 patients from our hospital were recruited for the trial between October 2010 and October 2015. They all provided signed informed consent. In brief, patients with STEMI who had undergone emergency PCI to treat an infarct-related artery (IRA) were eligible for inclusion if they had significant stenosis (70% by visual estimation) in at least 1 coronary artery other than the IRA (). They were randomized to the ComR or SelR arm using a pseudorandom number system within 48hours of the index PCI. Patients in the ComR group (n=154) were scheduled for a second PCI to treat all significant lesions prior to discharge. A pressure wire was not used to select which lesions to treat. Patients in the SelR group (n=152) underwent an exercise or dobutamine stress echocardiogram during the index admission. As per protocol, PCI was indicated for nonculprit lesions in patients with recurrent spontaneous myocardial ischemia, coronary lesions with evidence of low-load ischemia (≥ 120 beats per minute), or ischemia in more than 2 coronary segments (figure 1).
Flow diagram of the CROSS-AMI trial design. AMI, acute myocardial infarction; IRA, infarct-related artery; PCI, percutaneous coronary intervention. aDue to single-vessel coronary artery disease (n=904), multivessel index PCI (n=82), and suboptimal index PCI (n=30). bInadequate anatomy for PCI in lesions other than the IRA (n=245), significant disease in left truncus arteriosus (n=70), cardiogenic shock (n=73), severe comorbidity (=57), previous revascularization surgery (n=4), and AMI due to coronary stent thrombosis (n=12).
We performed a cost minimization analysis comparing the ComR and SelR strategies from the CROSS-AMI trial. The assumption in such analyses is that the strategies being compared are of similar effectiveness. The aim is thus to estimate and compare respective costs.
Index admission costs were estimated from the perspective of the public health care system in the autonomous community where our hospital is based. They were calculated as the sum of costs attributable to the emergency PCI, coronary angiography, repeat PCIs, and any diagnostic cardiology tests performed during the index admission. Follow-up costs were calculated as the sum of costs attributable to hospital stays during readmissions for a cardiac or noncardiac cause in the 12 months following discharge and any diagnostic cardiology tests or additional revascularization procedures performed during admission.
To estimate costs, we analyzed lengths of stay for the index admission and readmissions during follow-up. We also calculated the number of diagnostic and therapeutic cardiology procedures performed during these hospital stays. The costs were derived from official rates established for health care services provided by hospitals belonging to the public health care system in our autonomous community11 (table 1).
Hospitalization and cardiological procedure costs11
| Cost, € | |
|---|---|
| Hospitalization | |
| Cardiac or intensive care unit (per d) | 1142.47 |
| Conventional hospital ward (per d) | 528.95 |
| Noninvasive procedures | |
| Echocardiography | 321.64 |
| Exercise stress test | 310.76 |
| Exercise or pharmacological stress echocardiography | 377.67 |
| Myocardial perfusion with SPECT | 294.32 |
| Invasive procedures | |
| Coronary angiography | 1055.38 |
| Coronary angiography and balloon angioplasty | 3325.31 |
| Coronary angiography, balloon angioplasty, and stent placement | 6856.31 |
SPECT, single photon emission computed tomography.
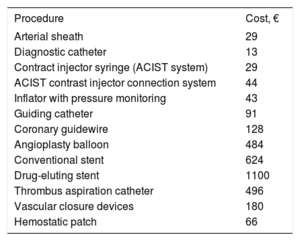
We also performed a detailed analysis of disposable material used. The rate system used in our health care system is generic and covers staffing costs, maintenance costs, and average material costs. It does not cover the cost of all the devices used for PCI (eg, sheaths, catheters, guidewires, balloons, stents). As per the trial protocol, a prospective record was made of all material used for PCIs performed during the index admission. The cost of this material, obtained from the purchasing department, was the price paid by the hospital (table 2).
Disposable material costs
| Procedure | Cost, € |
|---|---|
| Arterial sheath | 29 |
| Diagnostic catheter | 13 |
| Contract injector syringe (ACIST system) | 29 |
| ACIST contrast injector connection system | 44 |
| Inflator with pressure monitoring | 43 |
| Guiding catheter | 91 |
| Coronary guidewire | 128 |
| Angioplasty balloon | 484 |
| Conventional stent | 624 |
| Drug-eluting stent | 1100 |
| Thrombus aspiration catheter | 496 |
| Vascular closure devices | 180 |
| Hemostatic patch | 66 |
Quantitative variables are expressed as mean±standard deviation (SD), while categorical variables are described as absolute and relative frequencies. Costs are expressed as mean±SD costs per patient. Categorical variables were compared using the chi-square test or, where appropriate, the Fisher exact test, while quantitative variables were compared using the t test or, in the case of nonnormally distributed data, the Mann-Whitney U test. Statistical analyses were performed in SPSS version 24.0 (IBM). A P value of less than .05 was considered statistically significant for 2-tailed comparisons.
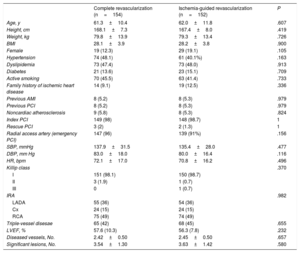
ResultsParticipantsThe baseline characteristics of the 306 patients randomized to the 2 trial arms are shown in table 3. There were no significant differences between the groups in terms of cardiovascular risk factors, medical history, or clinical presentation of myocardial infarction.
Patients’ baseline characteristics
| Complete revascularization (n=154) | Ischemia-guided revascularization (n=152) | P | |
|---|---|---|---|
| Age, y | 61.3±10.4 | 62.0±11.8 | .607 |
| Height, cm | 168.1±7.3 | 167.4±8.0 | .419 |
| Weight, kg | 79.8±13.9 | 79.3±13.4 | .726 |
| BMI | 28.1±3.9 | 28.2±3.8 | .900 |
| Female | 19 (12.3) | 29 (19.1) | .105 |
| Hypertension | 74 (48.1) | 61 (40.1%) | .163 |
| Dyslipidemia | 73 (47.4) | 73 (48.0) | .913 |
| Diabetes | 21 (13.6) | 23 (15.1) | .709 |
| Active smoking | 70 (45.5) | 63 (41.4) | .733 |
| Family history of ischemic heart disease | 14 (9.1) | 19 (12.5) | .336 |
| Previous AMI | 8 (5.2) | 8 (5.3) | .979 |
| Previous PCI | 8 (5.2) | 8 (5.3) | .979 |
| Noncardiac atherosclerosis | 9 (5.8) | 8 (5.3) | .824 |
| Index PCI | 149 (98) | 148 (98.7) | 1 |
| Rescue PCI | 3 (2) | 2 (1.3) | 1 |
| Radial access artery (emergency PCI) | 147 (96) | 139 (91%) | .156 |
| SBP, mmHg | 137.9±31.5 | 135.4±28.0 | .477 |
| DBP, mm Hg | 83.0±18.0 | 80.0±16.4 | .116 |
| HR, bpm | 72.1±17.0 | 70.8±16.2 | .496 |
| Killip class | .370 | ||
| I | 151 (98.1) | 150 (98.7) | |
| II | 3 (1.9) | 1 (0.7) | |
| III | 0 | 1 (0.7) | |
| IRA | .982 | ||
| LADA | 55 (36) | 54 (36) | |
| Cx | 24 (15) | 24 (15) | |
| RCA | 75 (49) | 74 (49) | |
| Triple-vessel disesae | 65 (42) | 68 (45) | .655 |
| LVEF, % | 57.6 (10.3) | 56.3 (7.8) | .232 |
| Diseased vessels, No. | 2.42±0.50 | 2.45±0.50 | .657 |
| Significant lesions, No. | 3.54±1.30 | 3.63±1.42 | .580 |
AMI, acute myocardial infarction; BMI, body mass index; Cx, circumflex artery; DBP, diastolic blood pressure; HR, heart rate; IRA, infarct-related artery; LADA, left anterior descending artery; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure.
Values are expressed as No. (%) or mean±standard deviation.
Of the 154 patients in the ComR arm, 152 (99%) underwent PCI for nonculprit lesions (147 required just 1 procedure, while 5 needed an additional 2). One patient requested to be switched to the SelR arm. The stress echocardiogram was negative and the patient was discharged without undergoing PCI. Another patient refused to undergo this procedure (figure 1).
Of the 152 patients in the SelR arm, one was switched to the ComR arm and treated with PCI, while another did not undergo a stress echocardiogram due to a mural thrombus. Two patients developed spontaneous recurrent ischemia before the stress echocardiogram and were treated with PCI as per protocol. Stress echocardiography was thus performed in 148 patients and showed extensive ischemia in 36, all of whom were treated with a second PCI. Of the remaining patients (64 with negative stress echocardiography results, 25 with inconclusive results, and 23 with results suggestive of mild ischemia), 1 underwent a second PCI before discharge because of spontaneous recurrent ischemia and 4 were referred for PCI by their cardiologists (figure 1).
EventsThe primary endpoint in the CROSS-AMI trial, a composite measure consisting of cardiovascular death, acute myocardial infarction, coronary revascularization, and readmission due to heart failure, occurred in 22 patients (14%) in the ComR group and 21 (14%) in the SelR group (hazard ratio, 0.95; 95% confidence interval [95%CI], 0.52-1.72; P=.85). There were no differences in risk for any of the components of the composite endpoint ().10
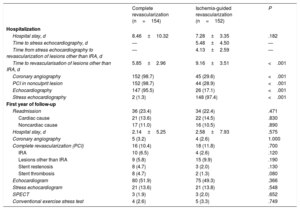
Cost comparisonDetails of lengths of stay and procedures and tests performed during the index admission and readmissions are shown for the ComR and SelR groups in table 4. The estimated costs are provided in table 5.
Procedures performed during index admission and readmissions during first year of follow-up
| Complete revascularization (n=154) | Ischemia-guided revascularization (n=152) | P | |
|---|---|---|---|
| Hospitalization | |||
| Hospital stay, d | 8.46±10.32 | 7.28±3.35 | .182 |
| Time to stress echocardiography, d | — | 5.48±4.50 | — |
| Time from stress echocardiography to revascularization of lesions other than IRA, d | — | 4.13±2.59 | — |
| Time to revascularisation of lesions other than IRA, d | 5.85±2.96 | 9.16±3.51 | <.001 |
| Coronary angiography | 152 (98.7) | 45 (29.6) | <.001 |
| PCI in nonculprit lesion | 152 (98.7) | 44 (28.9) | <.001 |
| Echocardiography | 147 (95.5) | 26 (17.1) | <.001 |
| Stress echocardiography | 2 (1.3) | 148 (97.4) | <.001 |
| First year of follow-up | |||
| Readmission | 36 (23.4) | 34 (22.4) | .471 |
| Cardiac cause | 21 (13.6) | 22 (14.5) | .830 |
| Noncardiac cause | 17 (11.0) | 16 (10.5) | .890 |
| Hospital stay, d | 2.14±5.25 | 2.58±7.93 | .575 |
| Coronary angiography | 5 (3.2) | 4 (2.6) | 1.000 |
| Complete revascularization (PCI) | 16 (10.4) | 18 (11.8) | .700 |
| IRA | 10 (6.5) | 4 (2.6) | .120 |
| Lesions other than IRA | 9 (5.8) | 15 (9.9) | .190 |
| Stent restenosis | 8 (4.7) | 3 (2.0) | .130 |
| Stent thrombosis | 8 (4.7) | 2 (1.3) | .080 |
| Echocardiogram | 80 (51.9) | 75 (49.3) | .366 |
| Stress echocardiogram | 21 (13.6) | 21 (13.8) | .548 |
| SPECT | 3 (1.9) | 3 (2.0) | .652 |
| Conventional exercise stress test | 4 (2.6) | 5 (3.3) | .749 |
IRA, infarct-related artery; PCI, percutaneous cutaneous intervention; SPECT, single photon emission computed tomography.
Values are expressed as No. (%) or mean±standard deviation.
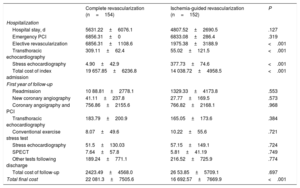
Costs (€) during index admission and readmissions during first year of follow-up
| Complete revascularization (n=154) | Ischemia-guided revascularization (n=152) | P | |
|---|---|---|---|
| Hospitalization | |||
| Hospital stay, d | 5631.22±6076.1 | 4807.52±2690.5 | .127 |
| Emergency PCI | 6856.31±0 | 6833.08±286.4 | .319 |
| Elective revascularization | 6856.31±1108.6 | 1975.38±3188.9 | <.001 |
| Transthoracic echocardiography | 309.11±62.4 | 55.02±121.5 | <.001 |
| Stress echocardiography | 4.90±42.9 | 377.73±74.6 | <.001 |
| Total cost of index admission | 19 657.85±6236.8 | 14 038.72±4958.5 | <.001 |
| First year of follow-up | |||
| Readmission | 10 88.81±2778.1 | 1329.33±4173.8 | .553 |
| New coronary angiography | 41.11±237.8 | 27.77±169.5 | .573 |
| Coronary angoigraphy and PCI | 756.86±2155.6 | 766.82±2168.1 | .968 |
| Transthoracic echocardiography | 183.79±200.9 | 165.05±173.6 | .384 |
| Conventional exercise stress test | 8.07±49.6 | 10.22±55.6 | .721 |
| Stress echocardiography | 51.5±130.03 | 57.15±149.1 | .724 |
| SPECT | 7.64±57.8 | 5.81±41.19 | .749 |
| Other tests following discharge | 189.24±771.1 | 216.52±725.9 | .774 |
| Total cost of follow-up | 2423.49±4568.0 | 26 53.85±5709.1 | .697 |
| Total final cost | 22 081.3±7505.6 | 16 692.57±7669.9 | <.001 |
PCI, percutaneous cutaneous intervention; SPECT, single photon emission computed tomography.
Values are expressed as No. (%) or mean±standard deviation.
No significant differences were observed between the ComR and SelR groups for length of stay during the index admission (8.46±10.32 vs 7.28±3.35 days, P=.182). Time to revascularization of nonculprit lesions was shorter in the ComR group than in the SelR group (5.9 vs 9.1 days, P<.001). In the SelR group, mean time from randomization to stress echocardiography was 5.5 days, while mean time from stress echocardiography to PCI was 4.13 days.
As mentioned, 98.7% of patients allocated to the ComR group underwent elective PCI for nonculprit lesions, compared with just 28.9% in the SelR group (P<.001). By contrast, stress echocardiography was performed in 97.4% of patients in SelR group but in just 1.3% of those in the ComR group (P<.001).
Transthoracic echocardiography was performed in 95.5% of ComR patients but in just 17.1% of SelR patients (P<.001).
Although the cost of emergency PCI during the index admission was similar for both groups (€6856.3 for ComR vs €6833 for SelR, P=.319), the cost of elective PCIs was significantly higher in the ComR group (€6856.3 vs €1975.4, P<.001). The cost of stress echocardiography, by contrast, was higher in the SelR group (€377.7 vs €4.9, P<.001).
The total estimated cost for the index admission was €19 657.9 in the ComR group vs €14 038.7 in the SelR group (P<.001). In other words, the SelR strategy yielded an estimated mean savings of €5619.13 (95%CI, €4350.6-€6887.7) per patient.
First year of follow-upReadmission rates in the 12 months following discharge were similar in the 2 groups (23.4% for ComR vs 22.4% for SelR, P=.471). The reasons for readmission included cardiac conditions (13.6% vs 14.5%, P=.830) and noncardiac conditions (11.0% vs 10.5%, P=.890). There were no significant differences between the groups in terms of readmission length of stay (2.14 vs 2.58 days, P=.575).
A comparable proportion of patients in both groups underwent noninvasive cardiological tests (eg, transthoracic echocardiography [51.9% vs 49.3%, P=.366] and stress echocardiography [13.6% vs 13.8%, P=.548]) and coronary revascularization (10.4% vs 11.8%, P=.700).
Costs attributable to readmission stays (€1088.8 vs €1329.3, P=.553), additional tests such as echocardiography, stress echocardiography, and coronary angiographies, and new PCIs were also similar in the 2 groups.
The total estimated cost of readmission in the first year of follow-up was €2423.5 for the ComR group and €2653.9 for the SelR group (P=.697). The overall cost of the ComR strategy was therefore significantly higher than that of the SelR strategy (€22 081.3 vs €16 692.6, P<.001).
Cost of disposable materialNo significant differences were observed between the ComR and SelR groups for the cost of disposable material used in the index PCI (€1643 vs €1687) (table 6). The cost of elective PCI material, however, was significantly higher in the ComR group (€3166 vs €896, P<.001). The total cost of disposable material was therefore €4810.4 in the ComR group vs €2557.0 in the SelR group (difference of €2253 [95%CI, €1794.2-€2712.2] per patient).
Cost (€) of disposable material
| Complete revascularization (n=154) | Ischemia-guided revascularization (n=152) | P | |
|---|---|---|---|
| Cost of disposable material for index PCI | 1643.76±931.9 | 1687.59±924.3 | .680 |
| Cost of disposable material for second PCI during index admission | 3166.68±1771.2 | 869.38±1617.0 | <.001 |
| Total cost of disposable material | 4810.44±2026.1 | 2556.96±2051.7 | <.001 |
PCI, percutaneous coronary intervention.
Values are expressed as No. (%) or mean±standard deviation.
In this economic evaluation of data from the CROSS-AMI trial from the perspective of the public health care service in our autonomous community, the mean cost of the ComR strategy during the index admission was significantly higher than that of the SelR strategy (€19 658 vs €14 039). In other words, the SelR strategy yielded an estimated mean savings of €5619 per patient, attributable to the performance of fewer PCIs during the index admission. No differences, by contrast, were detected for readmission costs in the 12-month follow-up period analyzed. When admission and readmission costs were combined, the ComR strategy cost an estimated €5388 more than the SelR strategy.
The differences in costs are largely due to differences in the proportion of patients treated with PCI for nonculprit lesions during the index admission (just 29% of patients in the SelR group compared with 99% of those in the ComR group). This observation is supported by our complementary analysis of the cost of disposable material for elective PCIs (nonculprit lesions), which was significantly higher in the ComR group than the SelR group (€3166 vs €869, P<.001). No differences were observed for disposable material used for emergency PCIs.
One of the main benefits claimed for complete revascularization in patients with STEMI and multivessel disease is that it reduces the likelihood of future clinical events, thus offsetting the initially higher costs of revascularization.4 No significant differences, however, were observed between the ComR and SelR groups for the occurrence of clinical events during the 12-month follow-up period.10 Readmission costs were thus similar for the 2 strategies, tipping the balance in favor of the less costly SelR strategy based on ischemia detection testing and against the more costly ComR strategy involving PCIs. Our findings show that the higher initial costs of the ComR strategy were not offset by savings expected from a reduction in clinical events during follow-up.
Times to stress echocardiography and revascularization in the CROSS-AMI trial10 can clearly be improved and may have influenced our results. The trial, however, was a pragmatic trial designed to reflect routine clinical practice, and therefore certain delays, influenced by multiple factors beyond the researchers’ control, were to be expected. Mean time to revascularization was significantly shorter in the ComR group than in the SelR group (5.85 vs 9.1 days, P<.001), but lengths of hospital stay and associated costs were comparable. In addition, the possible impact of delays was minimized because the mean time from randomization to performance of stress echocardiography in the SelR group was 5.5 days (similar to the time to revascularization in the ComR group) and just 29% of patients required a second PCI.
Numerous studies have analyzed the treatment of multivessel disease in STEMI,12,13 but just 1 of these—the Complete versus Culprit-Only Primary PCI trial (CvLPRIT)14—includes an economic evaluation.15 It is very difficult, however, to compare the results of this evaluation with ours due to differences in revascularization strategies, outcomes, and cost analysis methods. It could, however, be speculated that the results of the economic evaluation of the CvLPRIT trial might have been influenced by the inclusion of ischemia testing in the conservative arm. In addition, the findings of that evaluation raise numerous doubts. The authors estimated a 72% likelihood of complete revascularization being cost-effective at a willingness-to-pay threshold of £20 000 per quality-adjusted life year (QALY), raising questions about the effectiveness of this procedure in almost one-third of patients. In addition, QALYs were assessed using an indirect survey (EuroQoL 5-dimensional survey) administered to just 70% of patients. An additional drawback is the high percentage of missing values requiring multiple imputation, which carries a risk of bias and lack of precision.16 The authors detected no significant differences in the cost of the 2 treatment strategies during the index admission (£4890 in the complete revascularization group vs £4668 pounds in the IRA-only treatment group, P=.654). Based on our findings, higher costs would be expected in the former.
The CROSS-AMI trial, unlike the Compare-Acute17 and DANAMI-3-PRIMULTI18 trials, did not use invasive pressure-wire assessment of nonculprit lesions. We cannot compare costs between the trials, however, as no economic evaluations have been conducted for these 2 trials. This is unfortunate, as pressure wire measurement has been shown to be cost-effective in the setting of stable coronary disease.19,20 Invasive fractional flow reserve is a more costly technique than noninvasive stress echocardiography, and in addition, the cost of revascularization where necessary must also be considered. In the Compare-Acute trial, 55% of nonculprit lesions were treated, compared with just 28% of those in the CROSS-AMI SelR arm.
While complete revascularization has been shown to be superior to the treatment of culprit lesions only, 12 when it comes to “serious” events (death and myocardial infarction6), its true impact may have been overestimated due to the overly conservative treatment of nonculprit lesions.7 One recent proposal calls for an individualized approach to the treatment of multivessel disease in patients with STEMI, with decisions guided by clinical profile (age, frailty, comorbidities) and the severity, complexity, and relevance of nonculprit lesions. Economic considerations are also important.21 Our results show that SelR, based on elective revascularization only, is a more cost-effective strategy than ComR. It was associated with lower PCI costs during the index admission and similar costs during follow-up as the patients in both groups experienced a similar number of clinical events.
The optimal timing for the assessment and revascularization of nonculprit lesions in patients with STEMI and multivessel disease remains a matter of debate.7 In the CROSS-AMI trial, stress echocardiography and PCIs were performed during the index admission. Nonetheless, noninvasive assessment and revascularization within 4 to 6 weeks of discharge is a valid alternative for most patients with uncomplicated myocardial infarction, particularly considering that infarction causes physiological changes in coronary circulation.22 In the COMPLETE trial, the benefit of complete revascularization was independent of the timing of nonculprit lesion PCI.23
LimitationsOne of the main limitations of this study is the inclusion of just 306 patients (77% of the planned study population) due to the premature interruption of the CROSS-AMI.10 Nonetheless, based on the sensitivity analyses performed and the assumption of a similar rate of events, even if they all occurred in the same group, the likelihood of observing significant differences for the main endpoint with a full sample is remote.10
Selection bias due to exclusion criteria or analysis of baseline characteristics (eg, high frequency of Killip class I) cannot be ruled out. In all, 31% of candidates assessed for inclusion in the trial were finally recruited. While this may have affected the external validity of the trial, this inclusion rate of 31% is similar to rates reported for major clinical trials.13
Stress echocardiography provides more prognostic information than a conventional exercise stress test,24 but its interpretation may be more difficult and depends on the operator. That said, the hospitals that participated in the CROSS-AMI trial have extensive experience with stress echocardiography.25
Our findings might have varied with a longer follow-up period. This is unlikely, however, as no significant differences were observed between the 2 groups after 3 years in an extended follow-up evaluation. Nonetheless, it cannot be completely ruled out that differences would not have been detected after a follow-up period of more than 5 years.
Our use of current health care service rates limits the applicability of our findings to health care systems with comparable cost estimates. It should be noted that autonomous communities in Spain use different rate systems, making comparisons difficult. Another consideration is that potential cost variations in the future would alter our results.
The costs calculated for the follow-up period included cardiology procedures and treatments administered during readmissions, but not primary care visits, emergency room care, or other outpatient tests. Nonetheless, these costs would be unlikely to have a significant bearing as in the CvLPRIT15 trial, medical visits accounted for less than 10% of total costs.15
ConclusionsIn this economic evaluation nested within the CROSS-AMI clinical trial and conducted from the perspective of our health care system, the ComR strategy was significantly more expensive than the SelR strategy during the index admission. No significant differences were observed between the 2 groups for costs attributable to diagnostic and therapeutic procedures performed during hospital readmissions in the first year of follow-up.
The higher costs of the ComR strategy during the index admission were not thus offset by lower costs during the 12-month follow-up, probably due to the similar incidence of clinical events in the 2 groups.
In brief, in patients with STEMI and multivessel disease who undergo emergency PCI for an IRA, SelR would appear to be a more cost-effective strategy than ComR (treatment of all nonculprit lesions) for a follow-up period of 12 months, as it yielded similar clinical outcomes at a lower cost during this period.
FundingR. Estévez-Loureiro was the recipient of a Rio Hortega contract from the Instituto de Salud Carlos III during his participation in this project. The Instituto de Salud Carlos III did not participate in the design or conduct of this study.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest in relation to this publication.
- –
Multivessel disease is common in STEMI, has a worse prognosis, and is difficult to treat.
- –
Current recommendations call for consideration of complete revascularization of nonculprit lesions based on the findings of studies comparing this option with a conservative strategy involving the revascularization of nonculprit lesions.
- –
The CROSS-AMI trial, however, is the only clinical trial to date to compare 2 revascularization strategies.
- –
Economic evaluation studies are useful in complex situations such as this, particularly when resources are limited.
- –
The higher cost of complete revascularization compared with selective stress echocardiography–guided revascularization during the index admission was not offset by significant savings in a 12-month follow-up because the 2 groups experienced a similar number of clinical events.
- –
Selective stress echocardiography-guided revascularization in patients with STEMI and multivessel disease treated with emergency PCI of an IRA appears to be more cost-effective than complete revascularization during a 1-year follow-up period, as it achieved similar clinical outcomes at a lower cost.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.09.028