Substrate-based radiofrequency ablation of scar-related ventricular tachycardia (VT) is a standard procedure. Electroanatomical mapping can detect in-sinus rhythm heterogeneity in scar tissue and define conduction channels with abnormal and/or late potentials (LPs) targeted for VT ablation.1 Abolition of these LPs with noninducibility of VT has been shown to be the best marker for long-term follow-up success. Employing cardiac magnetic resonance imaging with late gadolinium enhancement (LGE-MRI) is useful when planning VT ablation; it can depict the conduction channels and increase the success of ablation.2
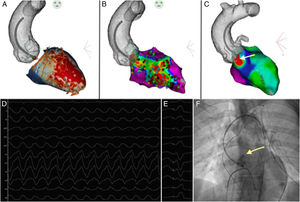
A 49-year-old man with a history of anteroseptal myocardial infarction was admitted to the emergency unit for dizziness and sweating. An electrocardiogram revealed a sustained monomorphic VT (VT-1) at 210 beats/minute. With a right bundle branch block QRS morphology and a left superior axis plus a precordial R-wave transition between V3 and V4, a mid-septal origin was suggested. The LGE-MRI identified a transmural scar involving the anteroseptal wall extending from the apex to the basal level. Images were processed using ADAS-VT (Galgo Medical, Spain) validated elsewhere.2 Scarring was detected in both the endocardial and epicardial layers. Several conduction channels that were potential substrates for VT were detected in both the endocardial (white) and epicardial (orange) zones at the basal, mid-septum, and apex areas (figure 1A).
Subsequently, radiofrequency VT ablation was performed using the CARTO3 (Biosense Webster, Johnson&Johnson, United States) mapping system. A cooled-type mapping and ablation catheter with a contact sensor (SmartTouch, Biosense Webster, United States) was advanced through the femoral artery, and an anatomical map of the ascending aorta and the aortic arch was completed. Using a previously acquired cardiac computed tomography angiography, an integration of the segmentation and the anatomical map of the aorta was performed using the navigation system. The left ventricle structure was shown by the LGE-MRI (previously integrated with the computed tomography). Endocardial electroanatomic mapping was performed using a PentaRay catheter, and LPs were found in the channels identified via LGE-MRI in the anteroseptal area (figure 1B). After a scheduled ventricular stimulation protocol, a sustained monomorphic VT (VT-2), different from the clinical VT (VT-1), was reproducibly induced (cycle length, 240ms). A left bundle branch block, V4 transition and inferior axis morphology suggested a basal septal origin, not mappable because the hemodynamic tolerance was poor. Substrate ablation following the previously described scar dechanneling technique2 was performed, and focal RF applications (40-50W) with an irrigated-tip catheter were delivered at the CC entrance, though abolishment of all LPs required additional radiofrequency applications inside the channels. Ablation was not carried out for the LPs next to the perihisian area because of a high risk of atrioventricular block. After complete substrate ablation, VT-2 remained inducible. In this case, LGE-MRI showed a predominance of epicardial channels in the basal septal portion close to the perihisian area, where some LPs in the endocardium were identified but not ablated. A pace map constructed using the PaSo module showed a QRS complex most similar to that in VT-2 between the right and left coronary cusps (arrow, figure 1C). The QRS morphology during tachycardia (VT-2, figure 1D) was compared with that during pace mapping (figure 1E), and at least 10 of the 12 electrocardiogram leads matched. Because ablation in endocardial channels had a high risk of AV block, radiofrequency was applied from the epicardium (aortic cusps). A selective angiogram through the ablation catheter then showed that the coronary arteries originated at safe distances (arrow at the level of the left coronary artery, figure 1F). Subsequently, radiofrequency at 20W was applied for 30seconds. Although endocardial channels were not removed, noninducibility was reached even with a complete stimulation protocol (600, 500 and 430ms basic pacing cycle length plus up to 3 extra stimuli using a coupling interval up to 200ms). With noninducibility, epicardial access via subxiphoid puncture was unnecessary. After 6 months, the patient remained VT-free.
On this basis, we propose an adjunctive approach to subxyphoid epicardial access when an epicardial basal anteroseptal substrate is identified. This area is very difficult to reach using conventional epicardial access. Indeed, epicardial access carries important risks that are avoidable using this approach. Moreover, in our case, endocardial ablation of that area could lead to atrioventricular block. Integration of the aortic anatomy, including the aortic cusps, from the computed tomography scan with the substrate from the LGE-MRI helped reveal that the epicardial substrate was accessible through the aortic cusps in this patient. Ablation in the aortic cusps must be performed carefully because it can be near important structures; therefore, low-energy applications and use of angiography could be beneficial when assessing distances to the coronary arteries prior to applying radiofrequency. A coronary angiogram is not always necessary if an intracardiac echocardiography catheter is used to show whether the ablation catheter tip is at a safe distance from the coronary arteries.
Ablation of VT from a coronary cusp has previously been described, but, to our knowledge, this is the first report of ablation at that site guided by image integration to determine the anatomy and amidst endocardial and epicardial ischemic scarring. Although this is an unusual location for VT ablation in an ischemic patient, our approach could be useful to avoid the risks of epicardial access when the localized epicardial substrate is near the aortic cusps.
A potential limitation of this case is that the mechanism of the VT-2 was not fully studied because of poor hemodynamic tolerance. Thus, a focal automatic mechanism within the cusp cannot be excluded, since ischemic and nonischemic cardiomyopathy may be present at the same time and the target area of ablation is more typical for the second one. This mechanism potentially explains why a single low-energy application was able to eliminate VT inducibility. This application was delivered after endocardial partial elimination of the conduction channels; therefore, both mechanisms (automatism and re-entry) might have been relevant in this patient.
FUNDINGThis work was supported by the Beca de la Sección de Estimulación Cardíaca de la SEC 2018; the European Union's Horizon 2020 Research and Innovation Programme [633196; CATCH ME project]; from Instituto de Salud Carlos III — Fondo de Investigaciones Sanitarias [PI16/00703 and PI16/00435]; Agència de gestió d’Ajuts Universitaris i de Recerca [AGAUR; 2017_SGR_1548]; and La MARATÓ-TV3 [20152730].
CONFLICTS OF INTERESTL. Mont reports activities as consultant, lecturer and advisory boards to Abbott Medical, Boston Scientific, Biosense Webster, Medtronic and Biotronik. He is shareholder of Galgo Medical, S.L. The other authors report no conflicts of interest relevant to the topic of this article.
.