We present excimer laser coronary atherectomy (ELCA) as an adjuvant therapy in percutaneous coronary intervention (PCI) when standard techniques fail (when crossing or expanding the lesion is impossible): the application of high-energy light provides a photochemical, photothermal, and photomechanical triple effect.1
Given that this technique is not widely used in our setting, there is a relative lack of data on its applications, effectiveness, and safety. We present our experience with ELCA over a 4-year period in the treatment of 31 lesions that underwent PCI (Table 1). We included lesions with specific indications for ELCA use as the first and only procedure (treatment of saphenous lesions and in-stent restenosis), as well as lesions with previous failed PCI attempts (52%).
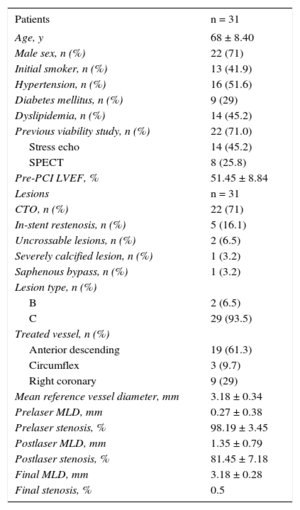
Clinical and Angiographic Characteristics
| Patients | n = 31 |
|---|---|
| Age, y | 68 ± 8.40 |
| Male sex, n (%) | 22 (71) |
| Initial smoker, n (%) | 13 (41.9) |
| Hypertension, n (%) | 16 (51.6) |
| Diabetes mellitus, n (%) | 9 (29) |
| Dyslipidemia, n (%) | 14 (45.2) |
| Previous viability study, n (%) | 22 (71.0) |
| Stress echo | 14 (45.2) |
| SPECT | 8 (25.8) |
| Pre-PCI LVEF, % | 51.45 ± 8.84 |
| Lesions | n = 31 |
| CTO, n (%) | 22 (71) |
| In-stent restenosis, n (%) | 5 (16.1) |
| Uncrossable lesions, n (%) | 2 (6.5) |
| Severely calcified lesion, n (%) | 1 (3.2) |
| Saphenous bypass, n (%) | 1 (3.2) |
| Lesion type, n (%) | |
| B | 2 (6.5) |
| C | 29 (93.5) |
| Treated vessel, n (%) | |
| Anterior descending | 19 (61.3) |
| Circumflex | 3 (9.7) |
| Right coronary | 9 (29) |
| Mean reference vessel diameter, mm | 3.18 ± 0.34 |
| Prelaser MLD, mm | 0.27 ± 0.38 |
| Prelaser stenosis, % | 98.19 ± 3.45 |
| Postlaser MLD, mm | 1.35 ± 0.79 |
| Postlaser stenosis, % | 81.45 ± 7.18 |
| Final MLD, mm | 3.18 ± 0.28 |
| Final stenosis, % | 0.5 |
CTO, chronic total occlusion; LVEF, left ventricular ejection fraction; MLD, mean lumen diameter; PCI, percutaneous coronary intervention; SPECT, single-photon emission computed tomography.
The lesions treated included 22 chronic total coronary occlusions (CTO), 5 in-stent restenoses (with underexpansion), 2 lesions that were “uncrossable” either with a guidewire or with any dilatation device after the guidewire was passed, 1 severely calcified lesion, and 1 saphenous bypass lesion. A 0.9mm catheter was used in 87.1% of the cases. The procedure was begun with the lowest energy level and frequency for each catheter, increasing progressively from 30 to 80mJ/mm2 and from 25 to 80Hz, respectively, until successful (the laser catheter crossing the lesion to the distal zone and/or dilatation of the lesion that was previously unexpandable with a balloon). The number of pulses used at each energy level was determined by the response. The catheter was advanced anterogradely from the proximal zone toward the lesion, with a maximum velocity of 0.5 to 1mm/s and a maximum pulse application time of 10seconds. Before each pulse, continuous perfusion was started with normal saline at 1mL/s for 10seconds as the laser was applied.
The mean fluence and frequency used were 66.43 ± 7.76 mJ/mm2 and 67.16 ± 8.77 Hz, respectively, with a mean number of pulses of 3.9 ± 1.47 (Table 2). The laser success rate at the first attempt was 93.5%. Independently of the type of lesion treated, the overall success rate of the procedure was 96.8%, with a final lumen diameter of 3.18 ± 0.28 mm and final residual stenosis of 0.5% (Table 1).
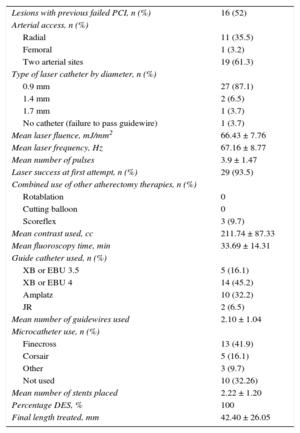
Technical Aspects of the Procedure
| Lesions with previous failed PCI, n (%) | 16 (52) |
| Arterial access, n (%) | |
| Radial | 11 (35.5) |
| Femoral | 1 (3.2) |
| Two arterial sites | 19 (61.3) |
| Type of laser catheter by diameter, n (%) | |
| 0.9 mm | 27 (87.1) |
| 1.4 mm | 2 (6.5) |
| 1.7 mm | 1 (3.7) |
| No catheter (failure to pass guidewire) | 1 (3.7) |
| Mean laser fluence, mJ/mm2 | 66.43 ± 7.76 |
| Mean laser frequency, Hz | 67.16 ± 8.77 |
| Mean number of pulses | 3.9 ± 1.47 |
| Laser success at first attempt, n (%) | 29 (93.5) |
| Combined use of other atherectomy therapies, n (%) | |
| Rotablation | 0 |
| Cutting balloon | 0 |
| Scoreflex | 3 (9.7) |
| Mean contrast used, cc | 211.74 ± 87.33 |
| Mean fluoroscopy time, min | 33.69 ± 14.31 |
| Guide catheter used, n (%) | |
| XB or EBU 3.5 | 5 (16.1) |
| XB or EBU 4 | 14 (45.2) |
| Amplatz | 10 (32.2) |
| JR | 2 (6.5) |
| Mean number of guidewires used | 2.10 ± 1.04 |
| Microcatheter use, n (%) | |
| Finecross | 13 (41.9) |
| Corsair | 5 (16.1) |
| Other | 3 (9.7) |
| Not used | 10 (32.26) |
| Mean number of stents placed | 2.22 ± 1.20 |
| Percentage DES, % | 100 |
| Final length treated, mm | 42.40 ± 26.05 |
DES, drug-eluting stent; PCI, percutaneous coronary intervention.
This is the largest published report to date on the use of ELCA as an adjuvant therapy in PCI of CTOs: 22 lesions were treated (71% of the total), compared with only 11 CTOs in the LEONARDO study2 (the largest series of ELCA-treated lesions) and 18 CTOs in the study by Fernandez et al.3 Due to its photothermal and photochemical effects, ELCA use in CTO allows molecular modification and changes in the physical structure of the fibrous capsule of the occlusion, allowing the device to be advanced to the distal bed. The success rate in this CTO subgroup was 95.45% (higher than that reported in previous literature, where it varied between 77% and 100%2,3). There was just one case of technique failure because it was impossible to pass the guidewire.
We must highlight the 100% success rate in the treatment of in-stent restenosis (16% of the total) without subsequent complications. These results are consistent with those of previous studies, such as the ELLEMENT registry.4
From a safety perspective, it should be noted that the first devices had a complication rate that was not insignificant, with a mean coronary perforation rate of 0.5% to 8%5 and a dissection rate of 7%.6 However, design improvements, such as the use of a flushing technique with normal saline to remove any residual blood or contrast, and the use of small diameter catheters (0.9mm), have enabled a reduction in the complication rate. In our study the complication rate was 3.2%, due to a single case of coronary artery perforation by the guidewire before the laser was used.
The results presented here allow us to recommend ELCA as a safe adjuvant therapy in complex PCI, when there is failure to cross or dilate the lesion as it significantly increases the success rate of the procedure.