We report an unusual case of late-onset phrenic nerve stimulation (PNS) in a super-responder to cardiac resynchronization therapy (CRT).
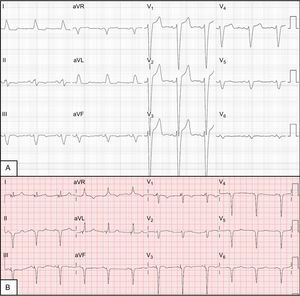
The patient was a 58-year-old woman with a history of hypertension and bronchial hyperreactivity. Four years earlier, she had been diagnosed with nonischemic dilated cardiomyopathy and left bundle-branch block (Figure 1A). She remained stable while receiving optimal medical treatment, with 40% left ventricular ejection fraction (LVEF) in New York Heart Association (NYHA) functional class II until her status worsened to NYHA III. An echocardiogram () revealed a spherical left ventricle (LV) with pronounced asynchrony, end-systolic volume of 128 mL, and LVEF (by Simpson method) of 26%. Consequently, a CRT device with defibrillator was implanted in June 2012. At that time, a bipolar lead was placed in a position with a long electrical delay (LV QRS interval of 180 ms). The LV capture threshold in bipolar pacing was 0.75 V at 0.4 ms, whereas impedance pacing was 460 Ω and R wave was 7 mV, without PNS (10 V output at 0.5 ms). Electrocardiogram showed simultaneous biventricular pacing, atrioventricular interval of 130 ms, and bipolar LV pacing with a QRS complex of 120 ms (baseline, 188 ms) and evident fusion between biventricular pacing and native conduction through the right branches (Figure 1B). Figure 2A shows the lead position on radiography in a posterolateral branch of the coronary sinus.
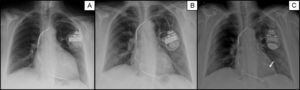
A: Posteroanterior chest radiography immediately after implantation of the defibrillator with resynchronization therapy. B: Chest radiography 9 months after implantation, when phrenic nerve stimulation appeared; the distance is clearly shorter between the distal pole of the left ventricle lead (A) and the edge of the cardiac silhouette; C: Chest radiography after implantation of the new quadripolar lead in the same vein; proximity between leads 2 and 3 (arrow) minimizes phrenic nerve stimulation.
The patient's clinical and echocardiographic progress was excellent. At 9 months postimplantation, the patient was in NYHA I and the echocardiogram showed disappearance of the spherical LV shape, noticeably decreased volumes (end-systolic volume, 32 mL), and normal LVEF (59%) (). However, shortly thereafter, she consulted for PNS in certain postures. The LV pacing threshold was still 0.75 V at 0.4 ms, there were no changes to the impedance or R wave detected, and LV lead dislodgement was ruled out by chest radiography (Figure 2B). The pacing configuration was changed from LV to pseudo-bipolar (proximal annulus/right ventricle), which led to disappearance of PNS.
Two years later, the patient consulted again for PNS. The radiologic and electric stability of the LV leads was rechecked, but no pacing configuration was able to prevent PNS at that time. A second operation was performed to remove the bipolar LV leads, and a quadripolar lead was implanted in the same bundle branch after confirmation that no other branches were suitable. The lead position for LV pacing was similar to the previous position (Figure 2C), but allowed more options for pacing configuration. Pacing by electrodes 2 and 3, which are very close to each other, had a threshold of 0.5 V at 0.4 ms and no PNS at 10 V and 0.5 ms. Eight months later, the patient had experienced no further symptoms of PNS and maintained good response to CRT.
Due to the close anatomic relationship between the phrenic nerve and LV, PNS is a common problem that limits CRT.1 In 80% of patients with PNS, it occurs close to the lateral and posterior branches of the coronary sinus2 and, therefore, often appears at the anatomic site considered optimal for resynchronization. Up to 35% of patients display it during implantation,3 which often makes it necessary to switch the lead sites to suboptimal positions for CRT. Around 15% of patients experience PNS during follow-up, but it usually appears in the first few weeks and rarely de novo after 6 months postimplantation.3 Pacing tests with high voltage outputs during implantation make it possible to avoid sites that produce PNS, even though this maneuver can only be used with the patient in the supine position. This explains the appearance of PNS shortly after implantation, when the patient adopted different postures in her daily life, even in the absence of lead dislodgement or microdislodgement, which is usually accompanied by an increased LV threshold.
In our patient, it is difficult to explain late-onset PNS in view of the stable radiologic position and lack of changes in electrical parameters. It appears that strong reverse remodeling with smaller LV and morphologic changes led to a progressive change in the anatomic relationships between the cardiac veins and the phrenic nerve, which would explain the late onset and progression of PNS until making it inevitable with programming changes. Implantation of a quadripolar lead (even in the same vein) with the electrodes very close together, made it possible to maintain CRT and avoid PNS.
The phenomenon of PNS due to CRT-induced reverse remodeling has not been described to date and could explain some cases of late onset during follow-up in responders. This association should be confirmed in future studies.