The term aberrant conduction refers to transient branch block not due to previous QRS abnormalities, accessory pathway conduction, or unwanted drug effects.1 The block can occur at any level of the His-Purkinje system and may be due to different mechanisms. Phase 3 block (tachycardia-dependent) is due to invasion of tissue during the effective refractory period and can be a physiological or pathological phenomenon. A special form of this block is acceleration-dependent block, which is due to changes in the heart rate. Phase 4 block (bradycardia-dependent or pause-dependent) is almost always pathological. It occurs after the end of the refractory period due to decreased membrane potential, because of increased His-Purkinje automaticity or partial depolarization of the myocardial lesion. The fourth and last aberrant mechanism is due to hidden conduction, which is defined as the propagation of an impulse within the specific conduction system and can only be recognized by its effect on the impulse, the interval, or the following cycles.2 As indicated by its name, this phenomenon cannot be observed on surface electrocardiogram (ECG).
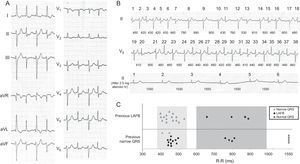
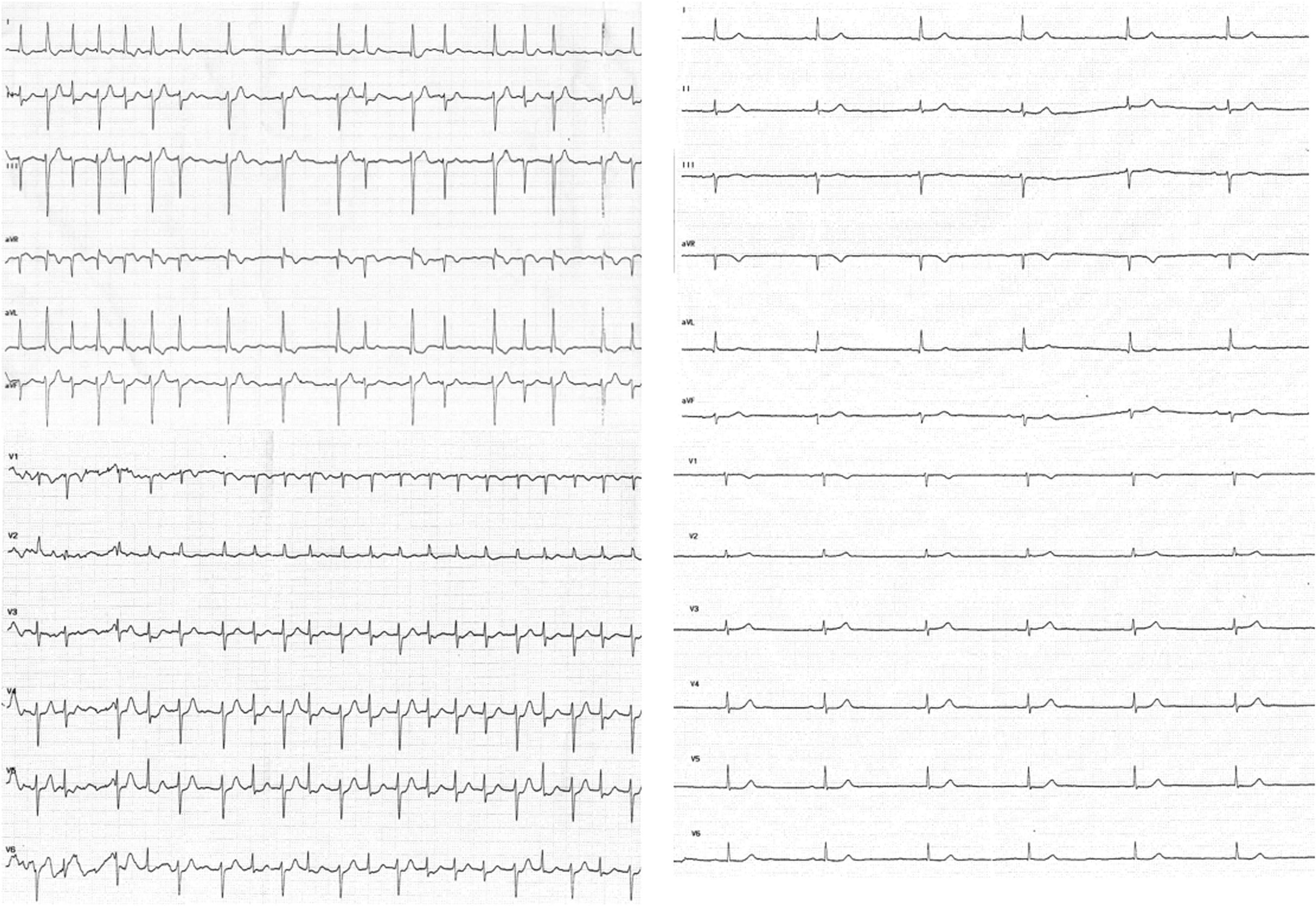
We present the case of an 86-year-old woman who was admitted to the emergency department for palpitations and dyspnea. Some years before, she had been assessed by a cardiologist for asymptomatic sinus bradycardia, for which she was not receiving treatment. Physical examination revealed irregular low-intensity heart sounds without murmurs and bibasal crackles with no other findings of interest. On admission, ECG showed atrial fibrillation with a ventricular response of around 100 bpm, with left anterior fascicular block (LAFB), alternating with beats with a narrower QRS complex (Figure 1A and Figure in the supplementary material). During her stay in the emergency department, the patient was administered 2.5 mg atenolol intravenously and achieved sinus rhythm at a rate of 39 bpm, with normalization of QRS morphology (Figure 1B and Figure in the supplementary material). The patient was discharged without antiarrhythmic medication. At 3 weeks, she was admitted with marked asthenia and documented sinus bradycardia at 35 bpm, for which she received a DDD pacemaker.
A: 12-lead electrocardiograph. B: Complete ECG tracing at admission. Beats 1–38 are consecutive despite being in 2 different leads (II and V5). Cycle lengths between beats are shown in milliseconds. The lower panel shows the QRS morphology after intravenous administration of 2.5 mg atenolol and conversion to sinus rhythm. C: The relationship of the QRS morphology with the cycle length and the morphology of the preceding beat. LAFB, left anterior fascicular block.
Careful analysis of the ECG obtained during the episode clearly showed 2 types of QRS: a) QRS with LAFB morphology (120ms), alternating with b) narrow QRS with small variations in axis and duration (90–100ms). Furthermore, at longer R–R intervals, beats always had LAFB morphology. Two areas can be clearly differentiated in Figure 1C, which compares the morphology of the QRS with the cycle length and the previous QRS complex.3 In zone 1 (R–R, 400–530 ms), the QRS morphology depends on the previous beat, (ie, if the previous beat is narrow, the following beat will have LAFB morphology). The only exceptions to this rule are beats 32–33, which could be explained by the penetration of the impulse in the supernormal conduction phase of the anterior fascicle.3,4 However, in zone 2 (R–R >600ms), the QRS complex always has LAFB morphology independently of the morphology of the previous beat, which is suggestive of bradycardia-dependent block. The curious aspect of this case is that, in contrast to what would be expected in this type of block, after a much longer R-R interval(>1500ms), the QRS becomes normal.
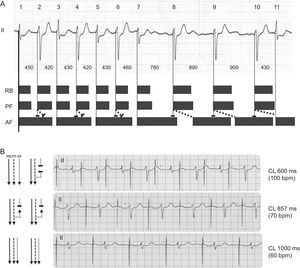
Figure 2 shows the proposed mechanism for these findings. With short R–R intervals (zone 1), an anterograde and retrograde block occurs in the anterior fascicle, which makes the following impulse able to conduct anterogradely since it has time to repolarize. In this way, the small variations in narrow QRS complexes could be explained by their occurring at different moments in their relative refractory period, with a higher or lower degree of latency (eg, beats 3 and 7, or 13 and 15). With very long cycle lengths, tissue recovery and permanent anterograde conduction take place. Cohen et al5 described this phenomenon at the end of the 1970s and called it pseudobradycardia-dependent branch block alternans (ie, a phase 3 block). For this to occur, the retrograde effective refractory period of the anterior fascicle should be less than the anterograde effective refractory period and thus favor hidden retrograde conduction.4
A. Proposed mechanism of the electrocardiographic findings. Black bars represent the theoretical effective refractory periods, and oblique dotted lines represent hidden retrograde interfascicular conduction. B. Confirmation of the mechanism with atrial pacing (AAI). AF, anterior fascicle; CL, cycle length; PF, posterior fascicle; RB, right branch.
We were able to confirm this mechanism (Figure 2B) because our patient had been implanted with a DDD pacemaker. Alternating LAFB was produced by AAI pacing at 100 bpm, at 70 bpm all beats were conducted with LAFB morphology, and at 60 bpm all beats were narrow, which confirmed tachycardia-dependent block.