Atrial fibrillation (AF) is the most common type of sustained arrhythmia in our setting.1 Cardioversion of AF persisting for more than 48hours requires prior oral anticoagulation to minimize the risk of thromboembolic events associated with the procedure.2 In this context, new oral anticoagulants (NOACs) have recently emerged as an alternative to the classic use of oral anticoagulation with vitamin K antagonists (VKAs), based on their efficacy and safety vs warfarin in cardioversion substudies of clinical trials on stroke prevention in nonvalvular AF.3–5 However, little is known about their safety in daily clinical practice,6 and there are no published data in our environment beyond the setting of randomized clinical trials.
We conducted a retrospective observational study of all cases of ambulatory cardioversion for AF performed at the Hospital Clínico Universitario de Valencia between 1 January 2012 and 1 April 2014. The type of anticoagulant therapy (VKAs or NOACs) was decided by the physician requesting cardioversion. In the case of VKAs, the patient required at least 2 international normalized ratio (INR) values ≥ 2 and at least 3 weeks of treatment. In the case of NOACs, patients required treatment for at least 3 weeks and were monitored to ensure adherence. All NOAC dosages and posology were required to match those used in the clinical trials that compared these drugs with warfarin in nonvalvular AF.3–5 We analyzed the incidence of thromboembolic events (stroke or systemic embolism [SE]) for 30 days after the procedure. Stroke was defined as the sudden appearance of a neurologic focal deficit located in the territory of a cerebral artery, as confirmed by imaging studies, and SE was defined as acute vascular occlusion in a limb or organ confirmed by imaging studies or surgery. The statistical analysis was performed using SPSS 17 (SPSS Inc.; Chicago, Illinois, United States).
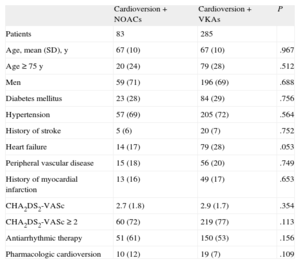
The study included 368 ambulatory cardioversions in 316 patients. A total of 83 (22.6%) procedures were performed in patients receiving NOACs (63 with dabigatran, 18 with rivaroxaban, and 2 with apixaban). The percentage of cardioversions performed with NOACs progressively increased (13.4% in 2012, 26% in 2013, and 35.1% in 2014; P<.01) compared VKAs. The baseline characteristics of patients according to the type of oral anticoagulant are shown in the Table. There were no significant differences in thromboembolic risk profile between patients treated with NOACs and those treated with VKAs (CHA2DS2-VASc, 2.9 vs 2.6; P=.27). There were no 30-day events in patients treated with NOACs; in contrast, there were 3 strokes (2 ischemic, 1 hemorrhagic) in patients treated with VKAs (P=.46). In both cases of ischemic stroke, an INR<2 was observed at admission. There were no reports of SE.
Baseline Characteristics of Patients According to Type of Anticoagulant Therapy
| Cardioversion+NOACs | Cardioversion+VKAs | P | |
| Patients | 83 | 285 | |
| Age, mean (SD), y | 67 (10) | 67 (10) | .967 |
| Age ≥ 75 y | 20 (24) | 79 (28) | .512 |
| Men | 59 (71) | 196 (69) | .688 |
| Diabetes mellitus | 23 (28) | 84 (29) | .756 |
| Hypertension | 57 (69) | 205 (72) | .564 |
| History of stroke | 5 (6) | 20 (7) | .752 |
| Heart failure | 14 (17) | 79 (28) | .053 |
| Peripheral vascular disease | 15 (18) | 56 (20) | .749 |
| History of myocardial infarction | 13 (16) | 49 (17) | .653 |
| CHA2DS2-VASc | 2.7 (1.8) | 2.9 (1.7) | .354 |
| CHA2DS2-VASc ≥ 2 | 60 (72) | 219 (77) | .113 |
| Antiarrhythmic therapy | 51 (61) | 150 (53) | .156 |
| Pharmacologic cardioversion | 10 (12) | 19 (7) | .109 |
NOACs, new oral anticoagulants; VKAs, vitamin K antagonists.
Unless otherwise indicated, the data are expressed as No. (%).
In recent years, substudies have been published on experience with cardioversion with NOACs compared with warfarin. In the RE-LY clinical trial, 672 cardioversions were performed in patients treated with dabigatran 150mg every 12 hours; 647 received a dose of 110mg every 12hours; and 664 received warfarin. There were no significant differences in the incidence of stroke or SE among the 3 treatment groups.3 In the ROCKET-AF study, even though scheduled cardioversion was an exclusion criterion for the clinical trial, 160 cardioversion or ablation procedures with rivaroxaban were performed, again with no new significant differences in stroke or SE compared with warfarin.4 A recent article reported on experience with cardioversion (412 with warfarin, 331 with apixaban) in the ARISTOTLE study, in which there were no 30-day thromboembolic events in either treatment group.5 However, few safety data are available apart from randomized clinical trial findings. Yadlapati et al6 have recently published their experience at Northwestern Memorial Hospital with a series of 53 patients treated with dabigatran or rivaroxaban. Our series resembles this study, as both were conducted in the context of daily clinical practice and found no significant differences between NOACs and VKAs in the incidence of stroke or SE after ambulatory cardioversion of AF, findings also consistent with those of the RE-LY, ROCKET-AF, and ARISTOTLE studies. The limitations of our study were mainly related to its retrospective design and the low number of procedures with NOACs. Experience with apixaban in our study was limited, primarily because the drug only became available recently.
According to our experience in daily clinical practice, ambulatory cardioversion of AF with NOACs is a procedure that is at least as safe as the usual pattern of using VKAs.