Concerns have been raised about higher rates of late stent thrombosis (ST) following implantation of drug-eluting stents.1 Localized hypersensitivity reactions were demonstrated in the vessel wall at autopsy in patients with late ST after implantation of first-generation drug-eluting stents. Hence, stents with a biocompatible coating were developed to minimize the vessel wall inflammation responsible for late events.2 The safety of titanium-nitride-oxide-coated stents based on a 316L stainless-steel platform has been demonstrated in unselected populations3 and in randomized clinical trials in acute coronary syndrome.4 Cobalt-chromium alloy has a superior radial strength that allows the development of ultra-thin struts but preserved radial force. In a prospective first-in-man observational study, we explored the 6-month clinical outcome of a titanium-nitride-oxide-coated stent based on a cobalt-chromium platform in de novo coronary lesions.
We enrolled 184 symptomatic patients with significant stenosis of a de novo lesion in a native coronary artery or a coronary bypass graft. Clinical presentation included stable angina and acute coronary syndrome. We excluded patients with heart failure, left ventricular ejection fraction < 30%, cardiogenic shock, renal impairment, prior target vessel revascularization, allergy to antithrombotic medications, active bleeding, and life expectancy < 12 months. The study was conducted according to the 1964 Declaration of Helsinki. Informed consent was obtained from all patients. The protocol was approved by our Ethics Committee. The OPTIMAX™ stent (Hexacath, Paris, France) is a thin-strut (81μm) stent, based on a cobalt-chromium platform with a twin helicoidal design. Titanium-nitride-oxide is coated on all surfaces of the stent. The stent is available in lengths of 7, 10, 13, 16, 19, 22, and 28mm, and in diameters of 2.25, 2.50, 2.75, 3.0, 3.5, and 4.0mm. Procedural success was defined as successful implantation of the stent with residual stenosis < 20% and final TIMI 3 flow, without dissection or thrombosis. Clinical success was defined as procedural success without in-hospital major adverse cardiac events (MACE). The primary endpoint was MACE at 6-months’ follow-up, defined as cardiac death, nonfatal myocardial infarction (MI), or ischemia-driven target lesion revascularization (TLR). Cardiac death was defined as death from cardiovascular causes or unknown cause. Myocardial infarction was diagnosed by persistent chest pain with an increase of CK-MB and/or troponin ≥ twice the upper reference limit. Target lesion revascularization was defined as a repeat intervention to treat significant (>50%) in-stent restenosis. Secondary endpoints included individual components of the primary endpoint, noncardiac death, ischemia-driven target vessel revascularization, and definite ST at 6-months’ follow-up. Stent thrombosis was adjudicated according to the Academic Research Consortium. Patients were prospectively followed up for 6 months. Follow-up coronary angiography was performed in patients developing recurrent symptoms during follow-up. An independent clinical event committee adjudicated all clinical events. Because of the observational design of the current study, no formal power calculation was performed.
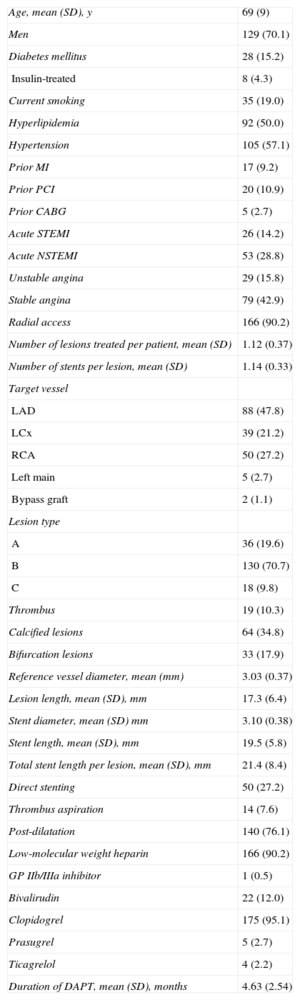
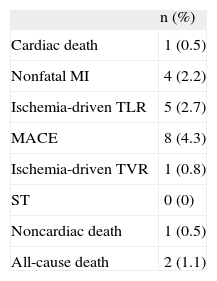
The mean age of the cohort was 69 (SD, 9) years; 70.1% were men. Patients presented with acute coronary syndrome in 57.1%, radial access was used in 90.2%, and complex (type B and C) lesions were treated in 80.5%. Baseline clinical and procedural characteristics are shown in Table 1. Procedural and clinical success occurred in 100% of the patients. All patients completed the 6-month clinical follow-up. The mean duration of follow-up was 198 (SD, 18) days. At 6-month follow-up, the primary endpoint of MACE occurred in 4.3%. No ST was observed. Clinical outcome at 6 months is summarized in Table 2.
Baseline Clinical and Procedural Characteristics (n=184)
| Age, mean (SD), y | 69 (9) |
| Men | 129 (70.1) |
| Diabetes mellitus | 28 (15.2) |
| Insulin-treated | 8 (4.3) |
| Current smoking | 35 (19.0) |
| Hyperlipidemia | 92 (50.0) |
| Hypertension | 105 (57.1) |
| Prior MI | 17 (9.2) |
| Prior PCI | 20 (10.9) |
| Prior CABG | 5 (2.7) |
| Acute STEMI | 26 (14.2) |
| Acute NSTEMI | 53 (28.8) |
| Unstable angina | 29 (15.8) |
| Stable angina | 79 (42.9) |
| Radial access | 166 (90.2) |
| Number of lesions treated per patient, mean (SD) | 1.12 (0.37) |
| Number of stents per lesion, mean (SD) | 1.14 (0.33) |
| Target vessel | |
| LAD | 88 (47.8) |
| LCx | 39 (21.2) |
| RCA | 50 (27.2) |
| Left main | 5 (2.7) |
| Bypass graft | 2 (1.1) |
| Lesion type | |
| A | 36 (19.6) |
| B | 130 (70.7) |
| C | 18 (9.8) |
| Thrombus | 19 (10.3) |
| Calcified lesions | 64 (34.8) |
| Bifurcation lesions | 33 (17.9) |
| Reference vessel diameter, mean (mm) | 3.03 (0.37) |
| Lesion length, mean (SD), mm | 17.3 (6.4) |
| Stent diameter, mean (SD) mm | 3.10 (0.38) |
| Stent length, mean (SD), mm | 19.5 (5.8) |
| Total stent length per lesion, mean (SD), mm | 21.4 (8.4) |
| Direct stenting | 50 (27.2) |
| Thrombus aspiration | 14 (7.6) |
| Post-dilatation | 140 (76.1) |
| Low-molecular weight heparin | 166 (90.2) |
| GP IIb/IIIa inhibitor | 1 (0.5) |
| Bivalirudin | 22 (12.0) |
| Clopidogrel | 175 (95.1) |
| Prasugrel | 5 (2.7) |
| Ticagrelol | 4 (2.2) |
| Duration of DAPT, mean (SD), months | 4.63 (2.54) |
CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; LAD, left anterior artery; LCx, left circumflex artery; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction.
Continuous variables are presented as mean (standard deviation), while categorical variables are presented as frequency (percentage).
Clinical Outcome at 6-months’ Follow-up (n = 184)
| n (%) | |
| Cardiac death | 1 (0.5) |
| Nonfatal MI | 4 (2.2) |
| Ischemia-driven TLR | 5 (2.7) |
| MACE | 8 (4.3) |
| Ischemia-driven TVR | 1 (0.8) |
| ST | 0 (0) |
| Noncardiac death | 1 (0.5) |
| All-cause death | 2 (1.1) |
MACE, major adverse cardiac events; MI, myocardial infarction; ST, stent thrombosis; TLR, target lesion revascularization; TVR, target vessel revascularization.
Over the past decade, titanium-nitride-oxide-coated stents based on 316L stainless-steel platform have shown a satisfactory clinical outcome in observational studies and randomized clinical trials.3,4 In a report by Karjalainen et al on 193 patients (212 Titan® stents), the 6-month rate of MACE was 6.7%, nonfatal MI 2.1%, and target vessel revascularization 6.2%; the TLR rate was 3.6%.5 Nevertheless, 46% of patients had a prior MI (vs 8.5% in the current study). Interestingly, no ST occurred at 6-months’ follow-up.5 In the TIBET registry, which enrolled 156 diabetic patients (197 Titan® stents), the 6-month rate of MACE was 10.3%, mainly driven by 7.1% TLR.6 The high TLR rate is comprehensible, because all patients were diabetic; moreover, 20.3% had a prior MI. Again, no ST occurred at 6 months.6 The main limitations of this study include selection bias to a favorable population, which may explain the 100% success rates and low rate of MI. The absence of angiographic follow-up may have contributed to the low TLR rate. We conclude that implantation of the OPTIMAX stent in de novo coronary lesions demonstrated excellent 6-month clinical outcomes, with a low rate of MACE and no ST.
FUNDINGThis work was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland.