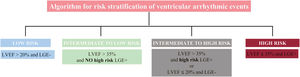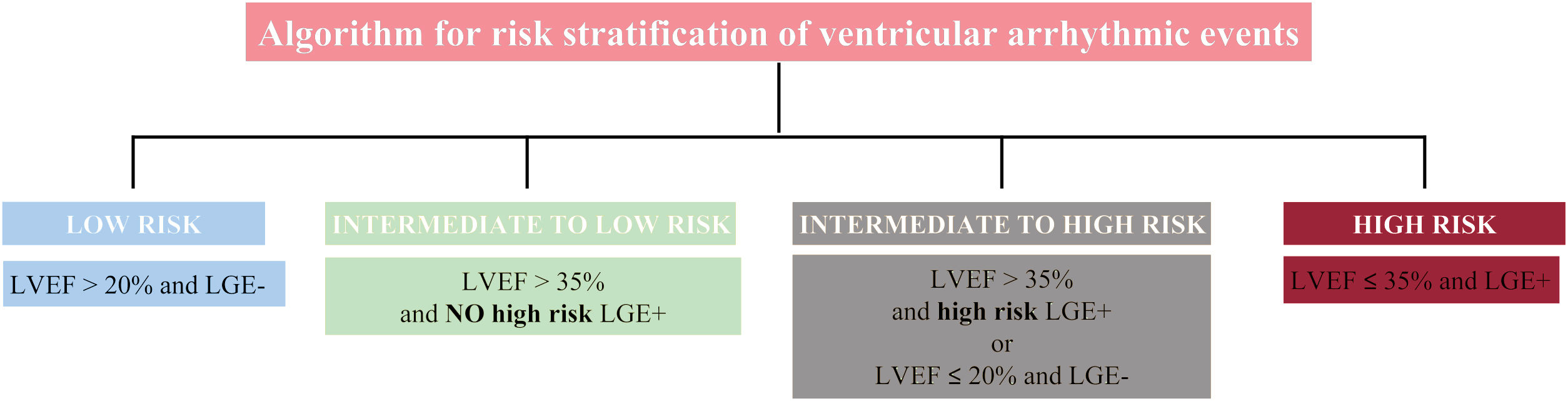
Left ventricular ejection fraction (LVEF) alone is not an accurate predictor of ventricular arrhythmic (VA) events in patients with nonischemic dilated cardiomyopathy (DCM). A new algorithm for risk stratification of VA was recently developed and tested in a cohort of patients with DCM,1 which combined late gadolinium enhancement (LGE) and LVEF strata by cardiac magnetic resonance (figure 1). This model improved risk stratification for VA compared with LVEF using a cutoff value of 35%. However, the new algorithm was evaluated in a retrospective observational cohort and is yet to be validated externally.
Algorithm for risk stratification of ventricular arrhythmia in nonischemic cardiomyopathy. The presence of epicardial, transmural or septal plus free-wall late gadolinium enhancement (LGE) was identified as high-risk LGE+. The remaining patterns were considered as NO high-risk LGE+. LVEF, left ventricular ejection fraction.
The aim of this study was to perform an external validation of this new algorithm in our prospective cohort of patients with DCM.
From 2014 to 2021, all patients with DCM were prospectively evaluated in our tertiary care hospital. DCM was defined as the presence of LVEF<45% (including true DCM with left ventricular dilatation and hypokinetic nondilated cardiomyopathy without left ventricular dilatation) in the absence of primary valve disease, hypertrophic cardiomyopathy, arrhythmogenic cardiomyopathy, cardiac amyloidosis, cardiac sarcoidosis, congenital heart disease, significant coronary artery disease (defined as >70% luminal stenosis in a major coronary artery or> 50% in the left main coronary artery) or a clinical history of myocardial infarction.
The study was approved by the local ethics committee. Informed consent was obtained from participants included in the study.
All patients underwent a 1.5 Tesla scanner cardiac magnetic resonance scan as part of the diagnostic workup. LVEF and left ventricular volumes were analyzed according to current guidelines.2 LGE was assessed visually and its extent was calculated as the number of affected myocardial segments. For the purpose of this study, a retrospective evaluation of LGE was performed, and the presence of epicardial, transmural or septal plus free-wall LGE was identified as high-risk LGE.1
An implantable cardioverter-defibrillator was implanted in patients with chronic symptomatic heart failure and LVEF <35% after 3 months of adequate medical therapy. The presence of LGE was not a criterion to implant an implantable cardioverter-defibrillator in our cohort.
Appropriate defibrillator therapies, sustained monomorphic ventricular tachycardia, sustained polymorphic ventricular tachycardia, resuscitated cardiac arrest and sudden death during follow-up were considered VA events, as defined in the original study.1
Statistical analyses were performed using Stata 14 (StataCorp LP, College Station, United States). Continuous variables are presented as mean±standard deviation. Categorical variables are expressed as frequency and percentage. Logistic regression and the Cox proportion hazards method were used to evaluate the distribution and incidence of VA within groups.
Differences in the discriminatory power among LVEF and the proposed algorithm were assessed by comparing their receiver-operating characteristic (ROC) curves. All tests were 2-sided, and differences were considered statistically significant at P values <.05.
The median age of our cohort (n=171) was 61±14.4 years and 65% were male. Mean LVEF was 29.8% (11.3). Most patients (> 90%) were treated with beta-blockers and renin-angiotensin-system blockers, and 68.7% with mineralocorticoid receptor antagonists. A total of 18% of patients had cardiac resynchronization therapy and 32.2% had an implantable cardioverter-defibrillator as primary prevention. The mean follow-up was 37.6±33.9 months.
During follow-up, 21 patients (12.3%) had a VA event. Of these, 17 patients had had an appropriate defibrillator therapy or ventricular tachycardia and 4 patients experienced sudden death.
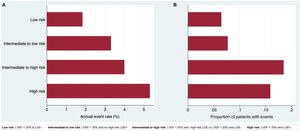
No significant differences (P=.909) were found in the incidence of VA among the different LVEF strata (< 20%; 21%-35%;> 35%). Conversely, both the presence of LGE (HR, 2.27; P=.089) and a high-risk pattern (HR, 2.986; P=.020) were significantly associated with VA. The distribution and incidence of VA events within the 4 groups of the algorithm were as follows (figure 2): a) low risk (n=62, 36.3%): annual event rate 1.84%; b) intermediate to low risk (n=13; 7.6%): annual event rate 3.31%; c) intermediate to high risk (n=27; 15.8%): annual event rate 4.00%; d) high risk (n=69; 40.35%): annual event rate 5.31%.
A comparison of the areas under the ROC curves between the new algorithm (0.660 [0.582-0.730]) and the LVEF <35% cutoff (0.501 [0.395-0.606]) showed a statistically significant superior predictive performance for the former (P=.025). Likewise, a comparison of the areas under the ROC curves between the new algorithm and the presence of LGE without considering LVEF (0.591 [0.513-0.665]) showed a trend toward higher predictive performance for the new algorithm (P=.066).
In our cohort, the algorithm had a 47.6% sensitivity, 79.9% specificity, very high negative predictive value (91.5%), and low positive predictive value (25%).
The present study is the first external validation of a proposed algorithm for risk stratification of VA in patients with DCM that includes LGE and LVEF information.1 Patients in our study had significantly lower LVEF than the original developing cohort (mean LVEF 29.8% vs 39%).1 As a consequence, patients meeting the high-risk category were more frequent in our study (40% vs 21%), and the incidence of VA was twice as high in our group (12.3% vs 6%). In our high-risk cohort, the new algorithm had a significantly higher accuracy for VA risk stratification than the LVEF <35% cutoff or the presence of LGE. However, the area under the ROC curve was lower than that obtained in the original population, and sensitivity and specificity values were fair. Therefore, the prediction of VA in DCM is challenging and quite uncertain. Interestingly, the negative predictive value was excellent.
The limitations of our work include the observational nature of the study and the limited number of patients included. Selection bias cannot be ruled out.
In conclusion, the new algorithm including LVEF and LGE patterns shows better performance than LVEF alone for VA discrimination and is particularly useful to identify low-risk patients. Further studies are necessary to improve risk stratification in patients with DCM and LVEF <35%.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSAll authors had access to the data and participated in the preparation of this manuscript. All authors contributed to the conceptualization of the study, data curation, formal analysis, investigation, methodology, validation, writing, and reviewing.
CONFLICTS OF INTERESTNone.
We thank Luis Ferrández for his contribution with English proofreading.