Tendon xanthomas (TX) are lipid deposits highly specific to familial hypercholesterolemia (FH). However, there is significant variability in their presentation among FH patients, primarily due to largely unknown causes. Lipoprotein(a) is a well-established independent risk factor for atherosclerotic cardiovascular disease in the general population as well as in FH. Given the wide variability of lipoprotein(a) among FH individuals and the likelihood that TX may result from a proatherogenic and proinflammatory condition, the objective of this study was to analyze the size of TX in the Achilles tendons of FH participants and the variables associated with their presence, including lipoprotein(a) concentration.
MethodsA cross-sectional study was conducted on 377 participants with a molecular diagnosis of heterozygous FH. Achilles tendon maximum thickness (ATMT) was measured using ultrasonography with standardized equipment and procedures. Demographic variables and lipid profiles were collected. A multivariate linear regression model using a log-Gaussian approach was used to predict TX size. Classical cardiovascular risk factors and lipoprotein(a) were included as explanatory variables.
ResultsThe mean low-density lipoprotein cholesterol level was 277mg/dL without lipid-lowering treatment, and the median ATMT was 5.50mm. We demonstrated that age, sex, low-density lipoprotein cholesterol, and lipoprotein(a) were independently associated with ATMT. However, these 4 variables did not account for most the interindividual variability observed (R2=0.205).
ConclusionsTX, a characteristic hallmark of FH, exhibit heterogeneity in their presentation. Interindividual variability can partially be explained by age, male sex, low-density lipoprotein cholesterol, and lipoprotein(a) but these factors account for only 20% of this heterogeneity.
Keywords
Familial hypercholesterolemia (FH) is an autosomal dominant-inherited genetic disorder of lipoprotein metabolism characterized by very high concentrations of low-density lipoprotein (LDL) cholesterol, increased risk of premature coronary heart disease (CHD), and superficial cholesterol-deposits on extravascular tissues such as tendon xanthomas (TX).1
TX are highly specific of FH in patients with high LDL cholesterol and are consequently an important diagnostic criterion in current guidelines.2,3 Clinically detected TX are typically present in one-third of patients with heterozygous FH (HeFH), starting in the second decade of life and increasing with age.4 The Achilles tendon is the most common location for TX to develop, and Achilles tendon ultrasonography increases the detection of TX to up to 75% of HeFH in adulthood.5 TX appear sonographically as one or more focal nodularities or anterior-posterior thickening of tendons. However, the prevalence of TX in HeFH is probably biased due to their high significance for the clinical diagnosis of FH.
Atherosclerotic plaques and xanthomas share many characteristics. Both are composed of collagen and foam cells, the latter derived from macrophages as a result of increased uptake of oxidized LDL particles. The lipid composition of TX consists of 55% free cholesterol, 28% cholesterol esters and 13% phospholipids, closely resembling fatty streaks and advanced atheromatous lesions in arteries.6,7 These similarities suggest related pathogenic mechanisms between TX and atherosclerotic coronary disease. Indeed, TX may be associated with a more than 3-fold higher risk of premature CHD among HeFH patients.4,8–10 However, this association is attenuated or disappears when adjusted for major cardiovascular risk factors, including LDL cholesterol.11,12
Another interesting similarity between TX and atherosclerotic cardiovascular disease is the interindividual variation in terms of their presence. Common cardiovascular risk factors as well as FH-specific factors, such as the type of mutation causing FH, are associated with CHD risk in HeFH and partially explain the CHD differences among HeFH.12 However, traditional risk factors and identical defects in the LDLR gene poorly explain the variability in TX. This suggests that the appearance of TX is controlled by other, as yet unidentified factors,2 and their identification may help to better characterize CHD risk in this population.
Lipoprotein(a) [Lp(a)] is a well-known independent risk factor for atherosclerotic cardiovascular disease in the general population as well as in HeFH.13 HeFH patients with high Lp(a) level (> 50mg/dL) exhibit the highest cardiovascular risk compared with those with low Lp(a) levels and the same null mutation.14 Given that Lp(a) varies widely among HeFH individuals15 and TX are probably the result of a proatherogenic and proinflammatory condition,16 we hypothesized that elevated Lp(a) concentration is especially important in the presence of TX.
The aim of this study was to analyze the presence and size of TX in Achilles tendons of patients with a genetic diagnosis of HeFH and the variables associated with their presentation, including the concentration of Lp(a). We considered Achilles tendon maximum thickness (ATMT) to be a continuous variable, similar to the approach used for atherosclerosis, and that participants with clinical TX were likely on the higher spectrum of the distribution.
METHODSWe conducted a cross-sectional study of participants with a well-identified molecular diagnosis of HeFH, in whom we analyzed the potential association of clinical and biochemical characteristics with Achilles TX.
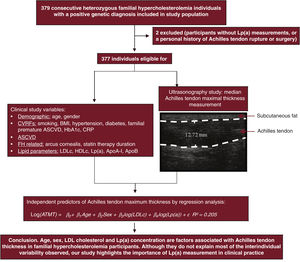
Consecutive HeFH individuals with a positive genetic diagnosis were selected from the lipid unit of Miguel Servet Hospital. We excluded participants without Lp(a) measurements, or a personal history of Achilles tendon rupture or surgery. In total, only 2 patients were excluded. Consequently, 377 participants fulfilled the study protocol and were included in the analysis.
Measurements of tendon xanthomasATMT was measured in the Achilles tendons using high-resolution ultrasonography with standardized equipment and operating procedures, as previously described.17
Clinical and laboratory parametersBlood samples were collected after 10hours of fasting and following discontinuation of any lipid-lowering treatment for at least 5 weeks, except for those participants with a personal history of cardiovascular disease or very high CVD risk. In the latter patients, past lipid values without lipid-lowering drugs were recorded if available, or lipid values were adjusted according to statin therapy.18 No patient had been under treatment with PCSK9 inhibitors at the time of the Achilles tendon ultrasonography and blood extraction, since PCSK9 inhibitors could potentially lower Lp(a) levels. All biochemical measurements were conducted in a central laboratory, as previously described.19 Lp(a) concentration was measured in fresh plasma samples by rate nephelometry using the LPAX reagent in conjunction with IMMAGE Immunochemistry Systems following the manufacturer's instructions. Four quality control samples were used daily, with a coefficient of variation<12% in all cases. Lp(a) results below the detection threshold were imputed as 0.5mg/dL, which is half of that threshold.
Samples from participants included in this study were provided by the Biobank of the Aragon Health System (PT17/0015/0039) with the approval of the Ethics and Scientific Committees.
In all participants with clinical suspicion of FH, LDLR (NM_000527.4), APOB (NM_000384.2), and PCSK9 (NM_174936.3) genes were studied using LIPOchip® (Progenika Biopharma-Grifols, Spain)20 or LIPID inCode® (GENinCode, Spain)21 platforms. These platforms include point mutations, large rearrangements, and copy number variations. Only participants with pathogenic or likely pathogenic mutations in canonical FH genes were included. The pathogenicity of the genetic variants was determined in accordance with the guidelines of the American College of Medical Genetics and Genomics.22
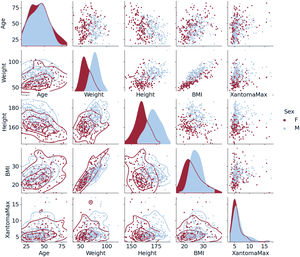
Data were analyzed using R Studio software, version 2022.07.2+576. We performed a descriptive analysis of quantitative variables with normal distribution and the results are reported as the mean±standard deviation. For quantitative variables with nonparametric distribution (Lp(a), triglycerides, C-reactive protein, maximum diameter of TX), the data are reported as the median [interquartile range]. The total number of cases and percentages were estimated for qualitative variables. The latter were compared using the Pearson chi-square test, mean values with the Student t-test, and median values with the nonparametric Mann-Whitney U test. A P value ≤.05 was considered statistically significant. Pair plots were used to explore potential associations among variables of interest. For the independent prediction of ATMT from demographic and lipid variables (table 1), a linear regression with a log-Gaussian model was used.
Characteristics of the familial hypercholesterolemia group
| Number of participants | 377 |
|---|---|
| Mean age, y | 43.3±14.1 |
| Female sex | 200 (53.1) |
| Ever smoked | 187 (49.6) |
| Smoking pack-years among ever smokers | 20.1±18.1 |
| Body mass index, kg/m2 | 25.3±4.36 |
| Arcus cornealis | 138 (36.6) |
| Systolic blood pressure, mmHg | 128±17.1 |
| Diastolic blood pressure, mmHg | 76.8±10.3 |
| Diabetes | 11 (2.9) |
| Hypertension | 48 (12.7) |
| ASCVD | 35 (9.3) |
| Age of ASCVD, y | 48±10.7 |
| Familial premature ASCVD | 138 (36.6) |
| Statin therapy duration, y | 6.14±2.08 |
| Total cholesterol, mg/dL | 357±68.4 |
| Median triglycerides, mg/dL | 97 [69.2-154] |
| LDL cholesterol, mg/dL | 277±66.9 |
| HDL cholesterol, mg/dL | 53.2±14.7 |
| Median lipoprotein(a), mg/dL | 25.4 [9.6-59.6] |
| Apolipoprotein A-I, mg/dL | 145±30.7 |
| Apolipoprotein B, mg/dL | 179±42.0 |
| Median C-reactive protein, mg/L | 1.50 [0.60-3.10] |
| HbA1c, % | 5.35±0.47 |
| Median Achilles tendon maximal thickness, mm | 5.50 [4.92-6.54] |
ASCVD denotes atherosclerotic cardiovascular disease; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Data are expressed as No. (%), mean±standard deviation or median [interquartile range].
The study group consisted of 377 HeFH participants with pathogenic (n=312, 82.8%) or likely pathogenic (n=65, 17.2%) variants in LDLR (n=338, 89.8%), APOB (n=15, 4.0%), PCSK9 (n=4, 1.1%) and p.Leu167del mutation in APOE (n=20, 5.3%). Their main clinical and biochemical characteristics are presented in table 1. The mean age of the participants was 43.3 years, with a slight predominance of women over men (53.1% vs 46.9%). The prevalence of atherosclerotic cardiovascular disease (ASCVD), hypertension, and diabetes was relatively low, with rates of 9.3%, 12.7%, and 2.9%, respectively. The mean LDL cholesterol level was 277mg/dL without lipid-lowering treatment, and the median ATMT was 5.50mm, ranging from 3.38 to 16.6mm (table 1).
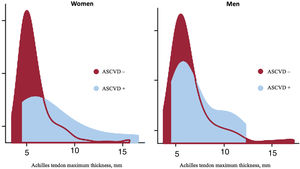
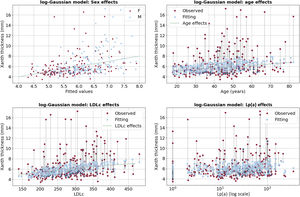
As expected, male participants were taller, heavier and had a higher prevalence of obesity compared with female participants. Similarly, obesity was more prevalent among older participants. Concerning ATMT, extremely large values were more prevalent among male participants, who are prone to be more obese (figure 1). The distribution of the ATMT showed a bimodal pattern in both men and women, with a substantial positive skew. In a small proportion of participants, ATMT exhibited significantly larger values, very far from the median/mean, particularly in those with ASCVD corresponding to thicker Achilles tendons (figure 2).
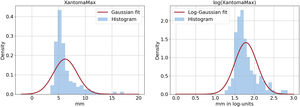
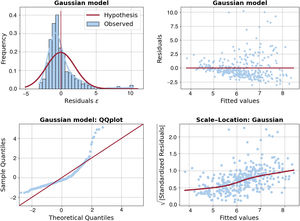
It is evident from the left panel of figure 3 that the Gaussian probability distribution function, which is the typical assumption in standard linear regression models, is not suitable for our data due to its symmetry and it fails to model outliers. A common solution to address the skewness problem is to apply log-transformation to the outcome variable, resulting in a target distribution that is closer to being symmetric (see the right panel of figure 3). Independent predictors of ATMT in the linear regression with log-Gaussian model are presented in table 2. Age, sex, LDL cholesterol and Lp(a) were found to be independently associated with ATMT, although these 4 variables explained only a small percentage of the variance in ATMT (R2=0.205). Additionally, the model's uncertainty increased as ATMT increased, as reflected in figure 4, where the residuals became larger as the size of the Achilles tendon increased. The remaining variables included in table 1, such as the duration of lipid-lowering treatment, did not exhibit a statistically significant association with ATMT.
Independent predictors of Achilles tendon maximum thickness by linear regression with log-Gaussian model
| Predictors | coef | std err | t | P> |t| | [0.025 | 0.975] |
|---|---|---|---|---|---|---|
| Intercept | −0.3123 | 0.331 | −0.944 | .346 | −0.963 | 0.339 |
| Sex (male) | 0.1143 | 0.028 | 4.092 | .000 | 0.059 | 0.169 |
| Age | 0.0045 | 0.001 | 4.255 | .000 | 0.002 | 0.007 |
| Log(LDLc) | 0.3171 | 0.061 | 5.173 | .000 | 0.197 | 0.438 |
| Log(Lp(a)) | 0.023 | 0.010 | 2.427 | .016 | 0.005 | 0.044 |
Coef, coefficient; LDLc, LDL cholesterol; std err, standard error; Lp(a): lipoprotein(a).
Regarding age effects, the largest residuals were positive (indicating underestimation) and occurred during middle age (from 45 to 60 years). This pattern suggests that a nonlinear U-shaped age effect could be beneficial for improving this model. As for LDL cholesterol and Lp(a), no clear pattern was observed in the residuals, which occurred across a range of lipid levels (figure 5).
Scatter plots of the outcome vs either the predictions (at the upper left panel) or each of the quantitative explanatory variables (for the rest of panels). The dotted vertical black lines in 3 of the panels show corresponding points in the scatter. In the age effects panel, a small jitter in the x-axis is added to avoid overlap of points.
LDLc, low density lipoprotein cholesterol; Lp(a), lipoprotein(a).
TX are a distinctive sign of FH that exhibit wide variability in their development. We found that age, male sex, LDL cholesterol, and Lp(a) concentration were associated with the ATMT measured by ultrasonography in HeFH participants with a confirmed genetic diagnosis. However, despite these 4 variables not fully explaining the observed interindividual variation (R2=0.205), Lp(a) measurement continues to be an important part of clinical practice in HeFH patients (figure 6).
Central illustration. Patient selection and determination of independent predictors of Achilles tendon xanthoma thickness using ultrasound in heterozygous familial hypercholesterolemia. The factors associated with Achilles tendon thickness in familial hypercholesterolemia participants were age, male sex, LDL cholesterol, and Lp(a) concentration. ApoA-I, apolipoprotein A-I; ApoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CRP, C-reactive protein; CVRFs, cardiovascular risk factors; HbA1c, glycated hemoglobin; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a).
The association between TX and CVD was described at the end of the 18th century.1 In the 1920s and 1930s, 2 Norwegian scientists, Francis Harbitz and Carl Müller, reported the microscopic characteristics of TX, their similarities with arteriosclerosis, and delineated a new disorder characterized by hereditary heart disease, xanthomatosis, and hypercholesterolemia, now called FH.23
In this study, our aim was to identify the variables associated with the presentation of TX in HeFH. Two points are especially important in our analysis of this association: first, we included only patients with a genetic diagnosis of HeFH, with pathogenic or likely pathogenic mutations in the canonical genes of FH. Furthermore, the trigger for the genetic study was suspicion of HeFH based on familial presentation of elevated LDL cholesterol concentrations, and patients were included consecutively regardless of the presence of cardiovascular disease or any clinical signs. Because TX is a powerful diagnostic criterion for FH, this study avoided selection based in the clinical diagnosis of HeFH and the bias of selecting a special subgroup within FH. The second important point of our methodology is the quantification of TX by high-resolution ultrasonography. Ultrasound allows not only a diagnosis of the presence of xanthomas when hypoechoic areas are found in the internal structure of the tendon but also allows quantitative analysis of the tendon size by measuring its anterior-posterior diameter.24 This enables a more precise analysis of the size of the Achilles tendon and analysis of the association of a quantitative variable.25 To avoid bias, the sonographers who performed the sonographic technique and measurement of the xanthomas were blinded to the patients’ clinical and biochemical characteristics.
Several important results of this study can be highlighted. First, the size of the Achilles tendon in HeFH is a continuous variable but has a bimodal distribution. This means that a percentage of the participants with HeFH have a greater predisposition to developing TX. The prevalence of TX varies among studies and largely depends on the diagnostic criteria of the disease, which in some cases includes the presence of TX, making it difficult to identify its true prevalence in the HeFH population. Additionally, studies that include detection by ultrasonography report a higher percentage of participants with TX. Furthermore, the diagnostic criteria for TX by ultrasonography are not well established and vary among studies. Using criteria that include the maximum diameter of the tendon, which is easily reproducible, the prevalence of xanthomas is around 40% of HeFH in adulthood.5
Second, the cause of TX is multifactorial and 4 variables seem to play an important role: age, male sex, LDL cholesterol and Lp(a) concentration. TX are infrequent before the age of 30 years and their prevalence increases until the age of 50 years and then stabilizes, suggesting that the triggering factors take time to act even in predisposed individuals. However, a group of HeFH individuals seem to be resistant to its development even at advanced ages. Predisposing and resistance factors do not seem to be related to the type of mutation responsible for FH or the LDL cholesterol concentration, as null allele participants also show wide heterogeneity in the development of TX. Men are more predisposed to the presence of TX, as occurs in the development of premature coronary disease, suggesting that the same factors that influence the development of coronary disease may also favor the occurrence of TX.11 The association with age, sex and LDL cholesterol was previously reported in clinically detected TX4 and was confirmed in a meta-analysis.7
Third, independently of classic risk factors, Lp(a) concentration is associated with the presence of TX in a linear fashion. This is consistent with the similarity in the pathogenesis between arteriosclerotic vascular plaques and the development of TX. Proatherogenic factors of Lp(a) include its proinflammatory role in transporting oxidized phospholipids and cholesterol inside the arterial wall and in modulating fibrinolysis. In fact, cultured monocytes from HeFH with TX have a higher predisposition to form foam cells in response to oxidized LDL than macrophages from patients without xanthomas.26,27 Furthermore, a decreased cholesterol efflux from macrophages to HDL has been reported in participants with xanthomas than in those without xanthomas.28 Likewise, SNVs associated with a differential response to inflammation are associated with the development of TX.29 The production of TX could be favored by inflammation, as evidenced by their preferential location in the Achilles tendon, a distal area of the body subjected to high mechanical load where factors that favor inflammation such as ischemia or traumatic stress could play an important role. However, C-reactive protein, a good marker of systemic inflammation, was not associated with the presence of TX in our study. Lipoprotein(a) levels remained without sex differences until age 50 years and were subsequently modestly higher in women30; in addition, it seems plausible that Lp(a) requires time to contribute to the development of TX. Therefore, a disaggregated analysis by sex is unlikely to fully explain sex variability in TX.
Finally, among all the clinical and analytical variables studied, only 4 seem to be independently associated with the presence of TX, and together they only explain around 20% of the variation in Achilles tendon size. This percentage is even lower in those participants with the most voluminous TX and therefore, in these patients, the uncertainty is even greater and indicates that we are still far from being able to explain the differences in the presence of TX in HeFH.
LimitationsOur study has several limitations. First, it is a cross-sectional study that includes some young participants and it is unknown whether they will develop TX in the future. Second, most of the participants were on on lipid-lowering treatment at the time of the study, some for long periods. Although the duration of statin use was not associated with the size of TX, we cannot definitively rule out its potential impact on our results. Nevertheless, statins do not substantially modify the concentration of Lp(a), suggesting that they may not play a significant role in the association found with this lipoprotein.
CONCLUSIONSTX, a characteristic sign of FH, exhibit heterogeneity in their presentation with a tendency toward bimodal presence in both sexes and a large positive skew. The variables associated with their thickness were age, male sex, LDL cholesterol, and Lp(a) concentration; however, these variables do not explain most of the interindividual variability observed. Our study highlights the importance of these 4 variables, including Lp(a) measurement, in the clinical practice in patients with FH.
FUNDINGThis study was supported by grants PI18/01777 and PI19/00694 from the Spanish Ministry of Economy and Competitiveness, CIBERCV and Gobierno de Aragón B-14. These projects are co-funded by Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) of the European Union “A way to make Europe”.
ETHICAL CONSIDERATIONSThis study complies with the Declaration of Helsinki and was approved by the local ethics committee (Comité de ética de la Investigación de la Comunidad Autónoma de Aragón). Written informed consent was obtained from all participants before their participation in this research study.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tool was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSP. Corredoira, F. Civeira and V. Marco-Benedi designed the study and wrote the English manuscript. A. Cenarro supervised the genetic analysis of participants with familial hypercholesterolemia. S. Peribáñez focused on fieldwork, collecting data and handling ethical issues. S. Olmos prepared the figures, conducted the statistical analysis and critically reviewed the study design.
CONFLICTS OF INTERESTNone declared.
- -
Tendon xanthomas are an important diagnostic criterion for familial hypercholesterolemia in current guidelines. Xanthomas and atherosclerotic plaques share many compositional characteristics, suggesting related pathogenic mechanisms.
- -
Common cardiovascular risk factors, including hypertension, diabetes, smoking, LDL cholesterol, as well as FH-specific factors such as the type of mutation causing FH, partially explain the CHD differences among HeFH; however, they poorly explain the variability in the presence of TX in FH participants.
- -
Lipoprotein(a) is a well-known independent risk factor for atherosclerotic cardiovascular disease not only in the general population but also in HeFH, contributing to a proatherogenic and proinflammatory condition.
- -
The distribution of the ATMT is a continuous variable but has a bimodal pattern. In a small proportion of participants, ATMT exhibited significantly larger values, particularly in those with ASCVD, corresponding to thicker Achilles tendons.
- -
An association between age, sex and LDL cholesterol with clinically detected TX was previously reported and confirmed in a meta-analysis. In our study, we found that Lp(a) was also independently associated with sonographically detected ATMT.
- -
Despite these findings, the variability in tendon xanthomas is not completely explained by these four variables, leaving up to 80% unexplained.
We wish to particularly acknowledge the patients and the Biobank of the Aragon Health System (PT17/0015/0039) integrated in the Spanish National Biobanks Network for their collaboration.