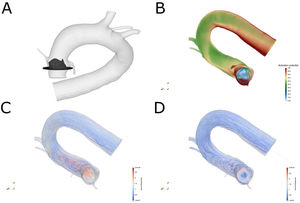
New commissural alignment techniques in transcatheter aortic valve implantation (TAVI) can facilitate coronary reaccess, if needed, and prevent coronary obstruction in valve-in-valve TAVI procedures. These techniques could also reduce central aortic regurgitation, gradient progression, and subclinical thrombosis, which would improve durability, all of which are crucial in low-risk patients. However, in specific patients it is impossible to determine differences between an implant with correct neocommissural alignment and one with misalignment. Computational fluid dynamics models have been widely validated for the cardiovascular system1 and could help to predict the impact of different degrees of commissural misalignment on transvalvular gradients2 and the degree of platelet activation in specific patients.3 Based on computed tomography (CT) scans of the patients included in this study, we estimated the central axis of the aorta and predicted the degree of clockwise/counterclockwise rotation required by the delivery system (self-expanding) or the prosthesis at crimping (balloon expandable) for correct commissural alignment using a previously described technique.4 After implantation, CT/angiography was used to assess the degree of alignment. Based on the patients’ anatomical and pressure data, computational fluid dynamics simulations were performed using the calculated velocity fields (figure 1A; ), with the valve fully open, by parametric study of the influence of the commissural alignment angle, rotating the valve in incremental 1-degree steps from perfect alignment (0°) up to 119°. These simulations were performed for two situations: a) an unrealistic completely uniform flow at the ventricular outflow tract, as simulated in current models (figure 1C); and (b) a helical flow similar to that used in MRI studies (figure 1D). The platelet activation model used was the same as the one validated in an in-vitro model of coronary bifurcation.3
A: three-dimensional representation of the valve in its implantation plane based on computed tomography of one of the patients and the valve designed by the authors. B: platelet activation potential calculated at peak systole for the linear inflow profile. C: current lines in the transvalvular flow model for the linear (uniform) velocity profile in the left ventricular outflow tract. D: streamlines in the transvalvular flow model with helical flow. WSS, wall shear stress.
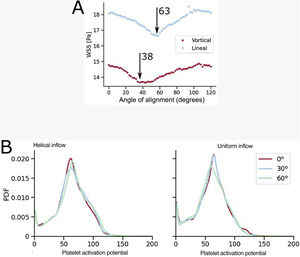
Forty patients with severe aortic stenosis, trileaflet aortic stenosis, severe calcification, and an aortic annulus area of 478±101mm2 (age, 81.4±4.9 years; STS risk score, 5.2±2.9) were treated with TAVI with commissural alignment. Ventricular function was 57.5±11.9% and mean gradient was 51.1±18.5mmHg, increasing to 6.3±2.1mmHg at 30 days, with no differences related to alignment (0% mortality). Mean commissural misalignment was 9.2° (ie, moderate; < 30°) in all patients. Significant differences in alignment were found between self-expanding prostheses (Acurate neo [Boston Scientific, EE. UU.] and Evolut [Medtronic, EE. UU.]: 7±3.2°; n=28) and balloon expandable prostheses (SAPIEN-3/Ultra [Edwards, EE. UU.] and MyVal [Meril, India]: 11.2°±4.9°; P=.001; n=12). Analysis of the 2 fluid dynamics simulations, as described above, (figure 2A), showed high sensitivity to ventricular outflow conditions, such that in the more realistic (helical) flow model an association was found between the absence of moderate or severe commissural misalignment (around 30° or less) and higher ventricular energy efficiency (lower line, red). However, in the uniform flow model, no association was found between better commissural alignment and reduced shear stress (upper line, blue). No differences were found between the 2 conditions (helical or uniform) in the degree of platelet activation according to previously validated models (figure 2B).
A: fluid dynamic simulation of energy efficiency of blood outflow through the valve (the lower the value, the higher the efficiency) as a function of the degree of alignment (shown in degrees) and the type of simulation (uniform or helical). The upper dashed line (blue) shows higher efficiency in severe misalignment (63°) if the flow is linear; the lower dashed line (red) shows higher overall efficiency and, in particular, in the absence of moderate or severe misalignment (38°) if the flow is helical. B: simulation of the degree of platelet activation as a function of the degree of commissural alignment (0°: perfect alignment; 30°: mild misalignment; 60°: severe misalignment), with no difference between helical (left) or linear (right) flow. PDF, probability density function.
Progressive understanding of the relevance of commissural alignment in TAVI has led to the development of custom software to help plan this procedure and to technical changes in the devices that make the procedure easier and faster. On the other hand, it is known that platelet activation takes place not only in situations of increased wall stress, such as aortic stenosis, but also in diseases with abnormal flows, such as bicuspid aortic valve disease.5 An association has been found between platelet activation and an increased risk of thrombotic and hemorrhagic phenomena.5 Previous studies have shown that computational models are quite accurate in predicting such activation; for example, they are able to predict thrombosis in coronary bifurcation stenting (figure 1B).3 The absence of differences in the degree of platelet activation as a function of the degree of misalignment, regardless of whether the flow is helical (figure 2B, left) or linear (figure 2B, right), suggests that the risk of clinical or subclinical thrombosis of the leaflets does not appear to differ as a function of the degree of misalignment. These models are based on purely mechanical assumptions (blood velocity fields), although there is a possibility that the development of more advanced models, which include elements of the coagulation cascade, will enable the detection of differences in thrombogenicity depending on commissural alignment.
On the other hand, the impact on ventricular energy efficiency suggests that, in each individual patient, the result in terms of gradients could differ depending on the degree of alignment. To detect this difference, the models used should simulate flows that are as realistic as possible (helical), given that current simulation laboratories use uniform flows through the prostheses under study.1 Should our findings be prospectively validated, it could be demonstrated that commissural alignment has an impact on prosthetic valve durability.
In conclusion, CT analysis prior to TAVI is highly accurate in predicting the rotation of the system to achieve correct commissural alignment, especially when using self-expanding prostheses. Furthermore, based on the same imaging tests, computational fluid dynamics analysis highlights the benefits of commissural alignment in taking advantage of the mechanical energy provided by the ventricular wall, but which can only be visualized in realistic (helical) simulations of flow in the outflow tract. We found that commissural alignment had no impact on the risk of leaflet thrombosis.
FUNDINGThis research has received funding from the Spanish Society of Cardiology (SEC) under grant number SEC/FEC-INV-CLI 21/023 and a FIS grant Nr PI21/01188 (Instituto de Salud Carlos III, Madrid, Spain).
AUTHORS’ CONTRIBUTIONSI. Amat-Santos and J. Sierra-Pallares designed and conducted the study. All authors approved the final version of the manuscript.
CONFLICTS OF INTERESTNone declared.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.01.012