Sodium-glucose cotransporter type 2 inhibitors (SGLT2i) have been associated with improved prognosis in patients with heart failure, but their impact on atrial arrhythmic (AA) and ventricular arrhythmic (VA) events is not fully understood.
MethodsThis multicenter retrospective study included patients with implantable cardioverter-defibrillators who initiated treatment with SGLT2i. AA and VA events were compared in 2 time periods for each patient: 1 year before and 1 year after starting SGLT2i.
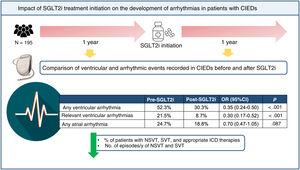
ResultsThe study included 195 patients (66.8 [61.3-73.1] years, 18.5% women). In the post-SGLT2i period, there was a reduction in the percentage of patients with any VA (pre: 52.3% vs post: 30.3%; P<.001) and clinically relevant VA (excluding nonsustained ventricular tachycardia) (pre: 21.5% vs post: 8.7%; P<.001). There was also a decrease in the number of episodes per patient/y of nonsustained ventricular tachycardia (pre: 2 (1-5) vs post: 1 (0-2); P<.001) and sustained ventricular tachycardia (pre: 1 (1-3) vs post: 0 (0-2); P=0.046). However, no differences were observed in the prevalence of AA (24.7% vs 18.8%; P=.117) or the burden of atrial fibrillation (pre: 0% (0-0.1) vs post: 0% (0-0); P=.097).
ConclusionsInitiation of SGLT2i treatment was associated with a decrease in the percentage of patients with relevant VA but this effect was not observed for AA.
Keywords
In recent years, sodium-glucose cotransporter 2 inhibitors (SGLT2is) have demonstrated prognostic improvements in various studies of patients with heart failure.1–4 These drugs are 1 of the 4 pillars of the recommended medical therapy in current clinical practice guidelines, together with beta-blockers, mineralocorticoid receptor antagonists (MRAs), and angiotensin receptor/neprilysin inhibitors (ARNIs).5,6
Several studies have linked the use of these drugs to reductions in sudden cardiac death (SCD) and in the incidences of atrial arrhythmia (AA) and atrial fibrillation (AF).7,8 However, their impact on arrhythmic events is incompletely understood and some studies have reached contradictory conclusions.9,10 This might be because only clinically symptomatic events are reported and not arrhythmic episodes that are asymptomatic but have prognostic value. Such episodes can only be detected in patients with continuous electrocardiographic monitoring. Thus far, just 1 observational study has assessed the association of SGLT2i treatment with reductions in AA and ventricular arrhythmia (VA) in patients with cardiac implantable electronic devices (CIEDs)11; the results indicated a reduction in AAs but not in VAs. Nonetheless, the study groups were not completely comparable because the patients receiving SGLT2i were younger and were more likely to have an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy (CRT) device. Accordingly, the objective of the current study was to assess the impact of SGLT2i initiation on the prevalence and incidence of AA and VA in a cohort of patients with an ICD by comparing events in 2 similar periods before and after drug initiation.
METHODSPopulationThe present retrospective multicenter study was conducted in 2 centers and included patients with an ICD with or without associated CRT who started treatment with an SGLT2i between January 2015 and January 2022. The study inclusion criteria were as follows: a) indication for treatment with SGLT2i (heart failure [HF] or diabetes mellitus); b) implanted with an ICD/CRT-ICD at least 1 year before SGLT2i initiation; and c) complete follow-up for at least 1 year after treatment initiation. The variable HF was defined as the presence of hospitalization for HF or an ambulatory New York Heart Association (NHYA) functional class > I under follow-up in the HF unit in each center. Follow-up was divided into 2 periods of equal length: the first period was 1 year prior to SGLT2i initiation (pre-SGLT2i) while the second period was 1 year after drug initiation (post-SGLT2i). Arrhythmic events were compared between the 2 periods. The study was approved by the local ethics committee of each center and all surviving patients at the time of analysis provided signed informed consent authorizing their participation.
Collection of arrhythmic eventsEvents were recorded in face-to-face consultations or via remote monitoring. All recorded episodes were analyzed by 2 electrophysiologists specialized in the reading of intracavitary tracings. If there were doubts about the type of event, the 2 electrophysiologists analyzed them together to reach a final diagnosis. For patients with remote monitoring, episodes occurring during the 2 periods were recorded using the different available platforms. For the remainder, episodes were collected from medical records. A nonsustained ventricular tachycardia (NSVT) event was defined as the presence of 3 or more ventricular complexes while sustained ventricular tachycardia (SVT) was defined as a ventricular tachycardia > 30seconds or requiring device therapy for resolution. A ventricular fibrillation (VF) event was defined as any ventricular tachyarrhythmia with a heart rate > 200 bpm. If the episode was still not resolved after an appropriate therapy, it was counted within the same episode. An appropriate therapy was defined as the presence of antitachycardia pacing (ATP) episodes or appropriate ICD discharge. We calculated the incidences of NSVT, SVT, ATP, VF, and appropriate and inappropriate ICD discharges, as well as the number of episodes per patient/y. VAs with a lower heart rate than the first programmed tachycardia window in the device were not recorded or included in the analysis.
For AAs, we collected atrial high-rate episodes and AF episodes of 30seconds to 6minutes, 6minutes to 24hours, and > 24hours. The variable any AA was defined as the presence of any episode, regardless of duration. Information was obtained on AF burden from patients with devices providing these data. To compare AA events between the 2 periods, patients with permanent AF were excluded.
EndpointsThe main endpoint of the study was assessment of the differences in the percentages of patients with relevant VAs (RVAs) and with any type of VA in the 2 periods. RVA was defined as any episode of SVT, VF, ATP, or appropriate ICD discharge. The variable any type of VA was defined as the presence of RVA or NSVT. The secondary study endpoints included the differences between the percentages of patients with NSVT, SVT, VF, ATP, or appropriate discharge and in the incidences of episodes per patient between the 2 periods.
Another end point related to AA events was defined as the difference in the percentage of patients with any AA episodes, as well as their duration. We also assessed differences in their incidence and in AF burden.
Statistical analysisContinuous variables are expressed as mean ± standard deviation or median [interquartile range] according to normality, which was determined using the Shapiro-Wilk test. Categorical variables are expressed as number and percentage and were compared using the chi-square test or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon matched-pair test while categorical variables before and after SGLT2i initiation were compared using the McNemar test. A sensitivity analysis was performed by excluding patients with events in the first 30 days after SGLT2i initiation, to allow a certain amount of time to pass before the drug took effect. To evaluate the influence of SGLT2i use on the reduction in arrhythmic events, we constructed a multivariable regression model using the generalized estimating equation method by including as confounding variables the various concomitant treatments in each period (angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin II receptor blockers [ARBs], ARNIs, beta-blockers, MRAs, amiodarone, and any antiarrhythmic agent). P < .05 was considered statistically significant. STATA version 15.1 was used for all analyses (STATA Corp, United States).
RESULTSPopulationOf the 442 patients with an ICD and under treatment with an SGLT2i, we excluded 247 due to an insufficient follow-up before or after the treatment. Ultimately, 195 patients were included (18.5% women; mean age, 66.8 [61.3 ± 73.1] years); 43.5% of the entire cohort had a diagnosis of AF before inclusion. Most (89.7%) had a clinical diagnosis of HF and the most prevalent etiology was ischemic (63%).
In addition, 132 of the patients (67.7%) had an ICD; the remainder had a CRT-ICD. Overall, 68.2% had a single-chamber device; of the CRT-ICD patients, 74.6% had an atrial lead. The implantation indication was primary prevention in 77.9% of cases. For 157 patients (80.5%), follow-up was conducted using remote monitoring in both periods; outpatient follow-up was performed for the remainder. Regarding the programming, 100% of the patients had a VF zone (212 ± 9.6 bpm), 95.4% had a rapid ventricular tachycardia (VT) zone (176 ± 7.6 bpm), and 5.6% had a slow VT zone (158 ± 30.3 bpm) (table 1).
Baseline characteristics of the cohort
| Variable | Population (n = 195) |
|---|---|
| Age, y | 66.8 [61.3-73.1] |
| Women | 36 (18.5) |
| Hypertension | 123 (63.1) |
| Dyslipidemia | 121 (62.1) |
| Diabetes mellitus | 54 (27.7) |
| COPD | 14 (7.2) |
| CKD | 21 (10.8) |
| Atrial fibrillation | 85 (43.5) |
| Paroxysmal atrial fibrillation | 42 (21.5) |
| Persistent atrial fibrillation | 2 (1.0) |
| Permanent atrial fibrillation | 41 (21.0) |
| Clinical heart failure | 175 (89.7) |
| Type of heart disease | |
| Ischemic | 109 (63.0) |
| Nonischemic dilated | 59 (29.5) |
| Valvular heart disease | 6 (3.5) |
| Infiltrative | 0 (0) |
| Cardiomyopathy | 3 (1.7) |
| Toxic | 3 (1.7) |
| Tachycardiomyopathy | 1 (0.6) |
| HF duration, mo | 53.0 [30.3-113.2] |
| LVEF, % | 30 [25-36] |
| Type of LVEF | |
| LVEF >50% | 11 (5.6) |
| LVEF 40%-50% | 17 (8.7) |
| LVEF < 40% | 167 (85.6) |
| Device type | |
| ICD | 132 (67.7) |
| CRT-ICD | 63 (32.3) |
| Time from device implantation, mo | 19.4 [8.2-38.8] |
| Primary prevention | 152 (77.9) |
| Secondary prevention | 43 (22.1) |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction.
Data are expressed as No. (%) or median [interquartile range].
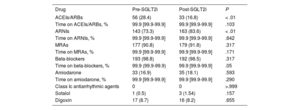
The treatment indication was HF in 175 patients (89.7%) and diabetes mellitus in the remainder; the most commonly used SGLT2i was dapagliflozin (71.8%). A higher percentage of patients were under treatment with an ACEI/ARB (28.4% vs 16.8%, P < .001) in the first period vs the second while a lower percentage of patients were being treated with ARNIs (73.3% vs 83.6%, P < .001). There were no differences between the 2 periods in the percentage of patients under treatment with MRAs, beta-blockers, amiodarone, antiarrhythmic agents for HF, sotalol, or digoxin or in the percentage of treatment time with each drug (table 2).
Drug therapy received in the 2 periods
| Drug | Pre-SGLT2i | Post-SGLT2i | P |
|---|---|---|---|
| ACEIs/ARBs | 56 (28.4) | 33 (16.8) | < .01 |
| Time on ACEIs/ARBs, % | 99.9 [99.9-99.9] | 99.9 [99.9-99.9] | .103 |
| ARNIs | 143 (73.3) | 163 (83.6) | < .01 |
| Time on ARNIs, % | 99.9 [99.9-99.9] | 99.9 [99.9-99.9] | .642 |
| MRAs | 177 (90.8) | 179 (91.8) | .317 |
| Time on MRAs, % | 99.9 [99.9-99.9] | 99.9 [99.9-99.9] | .171 |
| Beta-blockers | 193 (98.8) | 192 (98.5) | .317 |
| Time on beta-blockers, % | 99.9 (99.9-99.9) | 99.9 [99.9-99.9] | .05 |
| Amiodarone | 33 (16.9) | 35 (18.1) | .593 |
| Time on amiodarone, % | 99.9 [99.9-99.9] | 99.9 [99.9-99.9] | .290 |
| Class Ic antiarrhythmic agents | 0 | 0 | >.999 |
| Sotalol | 1 (0.5) | 3 (1.54) | .157 |
| Digoxin | 17 (8.7) | 16 (8.2) | .655 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ARNIs, angiotensin receptor-neprilysin inhibitors; MRAs, mineralocorticoid receptor antagonists; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
Data are expressed as No. (%) or median [interquartile range].
During follow-up, 2 patients (1.0%) underwent pulmonary vein isolation and 16 (8.2%) underwent VT ablation. AF was diagnosed in 12 patients (6.8%), 16 (8.2%) were admitted for arrhythmia, and 24 (12.3%) for decompensated HF. After a mean follow-up period of 30.8 (26.7-37.0) months, 8 patients (4.1%) died during the study period.
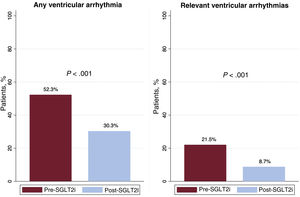
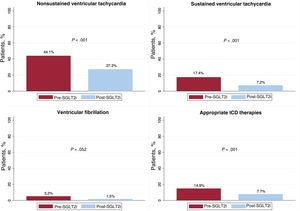
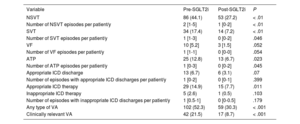
Ventricular arrhythmiasOf the complete cohort, 102 patients (52.3%) exhibited some type of VA in the period prior to SGLT2i initiation vs 59 (30.3%) after treatment initiation (P < .001). Of the patients who experienced a RVA, 42 (21.5%) had an episode in the first period vs 17 (8.7%) in the second (P < .001) (figure 1). This decrease in VA was due to a fall in the percentage of patients with NSVT (44.1% vs 27.2%, P < .001), SVT (17.4% vs 7.2%, P < .001), and APT (12.8% vs 6.7%, P = .023). Although decreases were detected in the number of patients with VF (5.2% vs 1.5%, P = .052) and with appropriate ICD discharge (6.7% vs 3.1%, P = .07), the differences were not significant. The number of patients with appropriate ICD therapy fell in the post-SGLT2i period (14.9% vs 7.7%, P = .011) (figure 2 and table 3). In addition, on multivariate analysis, the protective effect of the SGLT2is was maintained for both all types of VA (odds ratio [OR] = 0.35; 95% confidence interval [95%CI], 0.24-0.5; P < .001) and for RVAs (OR = 0.30; 95%CI, 0.17-0.52; P < .001).
Percentage of patients with ventricular arrhythmic events and their incidence in the 2 periods
| Variable | Pre-SGLT2i | Post-SGLT2i | P |
|---|---|---|---|
| NSVT | 86 (44.1) | 53 (27.2) | < .01 |
| Number of NSVT episodes per patient/y | 2 [1-5] | 1 [0-2] | < .01 |
| SVT | 34 (17.4) | 14 (7.2) | < .01 |
| Number of SVT episodes per patient/y | 1 [1-3] | 0 [0-2] | .046 |
| VF | 10 [5.2] | 3 [1.5] | .052 |
| Number of VF episodes per patient/y | 1 [1-1] | 0 [0-0] | .054 |
| ATP | 25 (12.8) | 13 (6.7) | .023 |
| Number of ATP episodes per patient/y | 1 [0-3] | 0 [0-2] | .045 |
| Appropriate ICD discharge | 13 (6.7) | 6 (3.1) | .07 |
| Number of episodes with appropriate ICD discharges per patient/y | 1 [0-2] | 0 [0-1] | .399 |
| Appropriate ICD therapy | 29 (14.9) | 15 (7.7) | .011 |
| Inappropriate ICD therapy | 5 (2.6) | 1 (0.5) | .103 |
| Number of episodes with inappropriate ICD discharges per patient/y | 1 [0.5-1] | 0 [0-0.5] | .179 |
| Any type of VA | 102 (52.3) | 59 (30.3) | < .001 |
| Clinically relevant VA | 42 (21.5) | 17 (8.7) | < .001 |
ATP, antitachycardia pacing; ICD, implantable cardioverter-defibrillator; NSVT, nonsustained ventricular tachycardia; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SVT, sustained ventricular tachycardia; VA, ventricular arrhythmia; VF, ventricular fibrillation.
Data are expressed as No. (%) or median [interquartile range].
In sensitivity analysis, after excluding patients with events within 30 days after drug initiation, we observed similar results for both any type of VA (41.9% vs 15.0%, P < .001) and RVAs (21,7% vs 8.3%, P < .001). These results were recorded both in patients with an ICD indication for primary prevention (any VA, 52.0% vs 28.9%, P < .001; RVA, 19.7% vs 6.6%, P < .001) and secondary prevention (any VA, 54.7% vs 35.7%, P = .033; RVA, 31.0% vs 16.8%, P = .034), as well as after the exclusion of patients who underwent VT ablation (VA, 51.4% vs 29.1%, P < .001; RVA, 19.0% vs 6.2%, P < .001). Given the higher number of patients with ARNIs in the post-SGLT2i period, we performed a subanalysis excluding patients who began treatment with this drug in the second period. Similar results were obtained (VA, 52.6% vs 31.4%, P < .001; RVAs, 20.6% vs 9.1%, P < .001).
Regarding the incidence of VA, the number of NSVT episodes per patient/y decreased in the post-SGLT2i period (before vs after, 2 [1-5] vs 1 [0-2], P < .001), as well as those of SVT (1 [1-3] vs 0 [0-2], P = .046) and ATP (1 [0-3] vs 0 [0-2], P = .045). There was no significant reduction in the incidence of VF episodes (1 [1-1] vs 0 [0-0], P = .054) or in the number of episodes with appropriate (1 [0-2] vs 0 [0-1], P = .399) and inappropriate (1 [0.5-1] vs 0 [0-0.5], P = .179) ICD discharges.
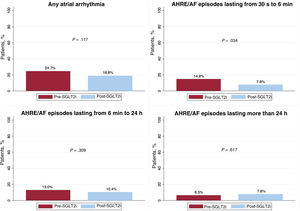
Atrial arrhythmiasOf the 85 patients (43.5%) with AF prior to study inclusion, 42 (49.4%) had paroxysmal AF, 2 (2.4%) had persistent AF, and 41 (48.2%) had permanent AF. Excluding the latter group, no decrease was recorded in the percentage of patients with AA events lasting from 6minutes to 24hours (13.0% vs 10.4%, P = .371) or those lasting more than 24hours (6.5% vs 7.9%, P = .617). However, there was a reduction in the percentage of patients with episodes lasting from 30seconds to 6minutes (14.9% vs 7.8%, P = .034). Among the patients who had some type of AA, there were also no differences in the incidences of AA episodes lasting from 30seconds to 6minutes (P = .143), from 6minutes to 24hours (P = .309), or for more than 24hours (P = .843). In addition, despite a fall in the percentage of patients with any AA, the difference was not statistically significant (24.7% vs 18.8%, P = 117) (figure 3). These results were maintained when multivariate analysis was conducted for any AA, even though there was a slight but nonsignificant protective effect (OR = 0.70, 95%CI, 0.47-1.05, P = .087). A total of 112 patients (57.4%) in the pre-SGLT2i period and 129 (66.2%) in the post-SGLT2i period had available data on AF burden. Analysis of these data failed to reveal significant differences between the 2 periods (P = .097) (table 4).
Percentage of patients with atrial arrhythmic events, incidence and AF burden, excluding patients with permanent AF
| Variable | Pre-SGLT2i (n = 154) | Post-SGLT2i (n = 154) | P |
|---|---|---|---|
| AHREs/AF episodes of 30 s-60 min | 23 (14.9) | 12 (7.8) | .034 |
| Number of AHRE/AF episodes of 30 s-60min per patient/y | 1 [0-4] | 0 [0-0] | .143 |
| AHREs/AF episodes of 6 min-24 h | 20 (13.0) | 16 (10.4) | .371 |
| Number of AHRE/AF episodes of 6 min-24 h per patient/y | 2 [0.5-7] | 1 [0-3] | .309 |
| AHREs/AF episodes of > 24 h | 10 (6.5) | 12 (7.8) | .617 |
| Number of AHRE/AF episodes of 6 min-24 h per patient/y | 1 [0-1] | 1 [0-2] | .843 |
| Any AHRE/AF episode | 38 (24.7) | 29 (18.8) | .117 |
| AF burden | 0 [0-0.1] | 0 [0-0] | .097 |
AF, atrial fibrillation; AHRE, atrial high-rate episode; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
Data are expressed as No. (%) or median [interquartile range].
This is the first study to assess the impact of treatment initiation with SGLT2i on the prevalence and incidence of AA and VA in the same cohort of patients with ICD in 2 follow-up periods, before and after SGLT2i initiation. The main findings of our study were the following: a) the number of patients with any type of VA or RVA decreased after SGLT2i initiation; b) this decrease was due to a fall in the number of patients with NSVT, SVT, and appropriate ICD therapies, as well as a drop in the incidences of NSVT and SVT; and c) there were no differences in the number of AA episodes or in AF burden (figure 4).
Central illustration. Impact of SGLT2i initiation on arrhythmic and ventricular events in patients with a CIED. CIED, cardiac implantable electronic device; NSVT, nonsustained ventricular tachycardia; OR, odds ratio; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SVT, sustained ventricular tachycardia.
Since the emergence of SGLT2is, substudies have linked their use to a lower incidence of AF and AA.12,13 In a meta-analysis of 34 randomized studies including more than 60 000 patients with diabetes, SGLT2is were associated with a 19% reduction in AA incidence.7 Another meta-analysis including patients with HF found a 25% decrease in the risk of AF events, both in patients with AF and without previous AF.14 Nonetheless, other studies have obtained contradictory results. In a clinical practice study of patients with diabetes, although SGLT2i treatment was associated with a reduction in new-onset arrhythmias, this result was not significant when AF and supraventricular arrhythmias events were separately evaluated.15 Along the same lines, a more recent meta-analysis of patients with HF found that SGLT2i treatment was not associated with a reduction in AA risk.8 However, one of the main limitations of these studies is that only clinically relevant AAs were reported and the arrhythmia burden is unknown. In the only study to assess events in patients with a CIED, Younis et al. retrospectively evaluated the effect of SGLT2is on AA burden.11 Their use was independently associated with a 15% reduction in the risk of AA and a fall in the number of events per year. However, as noted by the authors, although the results were adjusted by age, the patients receiving SGLT2i were younger, which means that the results need to be validated in prospective studies. In our study, despite a reduction in the number of patients with AA in the second period, the difference was not statistically significant (24.7% vs 18.8%, P = 117). In addition, no significant decrease was found in AF burden or in the incidence of AA episodes, despite a trend for protection in favor of SGLT2is (OR = 0.70; 95%CI, 0.47-105; P = .087). This lack of a benefit could be explained by the short follow-up period in our cohort and the low number of patients with an implanted atrial lead, which would have limited the monitoring of these events.
Analyses of the effect of SGLT2is on VA have linked these drugs to a reduction in SCD.7 A study of more than 150 000 patients detected a fall in the risk of SCD vs other antidiabetic agents, although the decrease was not significant.16 Another meta-analysis of 19 randomized studies also failed to find an association with a lower risk of VA.9 However, a meta-analysis of 22 studies including more than 50 000 patients did find a reduction in VT risk, but not in cardiac arrest.10 These discrepancies could be due to the heterogeneity of the included studies, as well as the low number of VAs recorded. Furthermore, a subanalysis of the DAPA-HF study reported an association between dapagliflozin therapy and a lower risk of the composite event of major VA, cardiac arrest, and SCD.17 Nonetheless, this effect was not found in patients with a CIED or after separate analysis of VAs. Along these lines, a recent meta-analysis by Oates et al.8 reported a decrease in SCD risk, but not in sustained VA.
However, as with the assessment of AAs, these studies only reported clinically relevant VAs and not asymptomatic VAs. In our study, the drop in VA was due to reductions in the percentages of patients with NSVT and SVT and in their incidence. These events are normally asymptomatic, particularly if they are effectively treated with APT; however, their onset and burden are associated with a worse prognosis.18 Moreover, and in contrast to the findings of our cohort, the study by Younis et al. failed to detect a reduction in VA risk, despite finding a fall in mortality and AA risk. These discrepancies could be due to a higher percentage of patients with ICDs in our cohort, with higher arrhythmic risk, a situation reflected in the higher percentage of events recorded vs that study.
From the pathophysiological perspective, several SGLT2i mechanisms have been described that could exert antiarrhythmic properties. First, SGLT2is have been reported to inhibit Ca2+ currents by reducing Ca2+ kinase II/calmodulin-dependent activity, which decreases the release of Ca2+ from the sarcoplasmic reticulum and thereby reduces arrhythmogenesis due to delayed depolarizations.19 The inhibition of late sodium currents has also been studied in murine models.20 In addition, reverse remodeling and decreased interstitial fibrosis have been related to microre-entrant and macrore-entrant phenomena.21,22 This effect, as well as the diuretic effects of SGLT2is, would reduce intracavitary pressures, which would decrease the parietal stress associated with the genesis of arrhythmic events.23 Finally, they might have a modulatory effect on the autonomic nervous system in patients with HF.24 Preclinical studies in mice have detected a possible inhibitory effect of the sympathetic nervous system due to a reduction in the renal concentrations of tyrosine hydroxylase and renal and cardiac norepinephrine.25 In addition, in the EMBODY trial, which randomized empagliflozin or placebo to 105 patients with diabetes after an acute myocardial infarction,26 there was improved autonomic nervous system activity, as evidenced by higher heart rate variability with SGLT2i, reflecting a greater parasympathetic balance.
Despite the above, much remains unknown concerning the effects of these drugs on arrhythmic events. However, various randomized studies are underway, such as the ERASe (NCT04600921)27 and DAPA-AF (NCT04792190)28 trials, which are assessing the impact of ertugliflozin and dapagliflozin on arrhythmic events in patients with a CIED.
LimitationsOur study contains certain limitations due to its design and retrospective nature. One of the main limitations is inherent to the selection bias caused by the exclusion of patients with at least 1 year of follow-up, which excludes those who died during this period. For this reason, the conclusions of this study cannot be generalized to patients with a worse functional class or who die soon after SGLT2i initiation. In addition, upon application of the recommendations of the latest clinical practice guidelines, the second period showed an increase in the proportion of patients under treatment with ARNI, a drug that has been associated with a decrease in arrhythmic events.29 However, a subanalysis excluding patients who switched medication reached the same conclusions, despite the sample size decrease. Furthermore, the treatment for HF was defined categorically and dose adjustments were not considered, meaning that the impact of the total dose on the outcomes could not be evaluated. In addition, the left ventricular ejection fraction was recorded at study inclusion and conclusions could not be reached regarding the dynamic changes during this period. Moreover, 19.5% of the cohort did not have complete remote monitoring and data may have been lost from these patients; however, data collection in the clinic was exhaustive and just 9.7% of patients had no remote monitoring in either period. Additionally, no analysis by sex was performed due to the low percentage of women (18.5%) included in the study. Finally, follow-up after SGLT2i initiation had a duration of 1 year, meaning that longer-term conclusions could not be made.
CONCLUSIONSIn conclusion, SGLT2i initiation in our cohort patients with an ICD or CRT-ICD was associated with a reduction in VAs and RVAs vs the pretreatment period. This reduction was due to falls in the percentages of patients with NSVT, SVT, and appropriate ICD therapies and in the incidences of NSVT and SVT. SGLT2i initiation was not accompanied by a reduction in AA in our study. However, prospective randomized studies are required to verify these conclusions.
- -
Observational studies have linked SGLT2i use with a lower incidence of atrial fibrillation and sudden cardiac death.
- -
SGLT2is may exert an antiarrhythmic effect and could decrease the risk of relevant ventricular arrhythmias in patients with cardiac implantable devices.
None.
ETHICAL CONSIDERATIONSThe study was approved by the local ethics committee of each center and all surviving patients at the time of analysis provided signed informed consent authorizing their participation.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEThe authors of this manuscript declare that they did not use any artificial intelligence tool in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONSC. Minguito-Carazo, E. Sánchez Muñoz, M. Rodríguez Mañero, J.L. Martínez-Sande, M.L. Fidalgo Andrés, J. García Seara, J.M. González Rebollo, M. Rodríguez Santamarta, L. González Melchor, T. González Ferrero, L. Romero Roche, J.A. Fernández López, and E. Tundidor Sanz contributed to the data collection.
C. Minguito-Carazo and E. Sánchez Muñoz contributed to the statistical analysis.
C. Minguito-Carazo and E. Sánchez Muñoz contributed to the manuscript drafting.
C. Minguito-Carazo, E. Sánchez Muñoz, M. Rodríguez Mañero, J.L. Martínez-Sande, M.L. Fidalgo Andrés, J. García Seara, J.M. González Rebollo, M. Rodríguez Santamarta, L. González Melchor, T. González Ferrero, L. Romero Roche, J.A. Fernández López, E. Tundidor Sanz, F. Fernández Vázquez, and J.R. González-Juanatey contributed to the manuscript revision.
CONFLICTS OF INTERESTNo conflicts of interest.
The authors appreciate the efforts of the nursing staff in charge of collecting arrhythmic event data in the clinic from the remote monitoring devices.