Death due to cardiogenic shock is still high (50%-80%) despite early coronary revascularization, intra-aortic balloon counterpulsation, and short-term mechanical ventricular assist devices (VADs; extracorporeal membrane oxygenator or Levitronix®).
One recently approved short-term percutaneous VAD, the Impella CP®, provides a theoretical flow of up to 4 L.
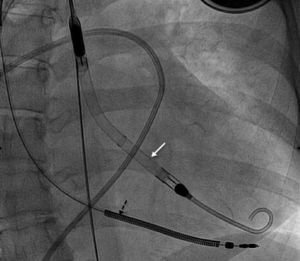
We report the case of a 37-year-old woman with cardiomyopathy after childhood thoracic chemotherapy and radiotherapy who had spent 6 months on the elective heart transplant list due to advanced heart failure. When the patient was admitted with refractory cardiogenic shock despite inotropic therapy (INTERMACS level 2), it was decided to implant a VAD as a bridge to heart transplant. However, due to the history of thoracic irradiation, reduced size of the left ventricle (46mL), and severe dysfunction of the right ventricle, she was a suboptimal candidate for a surgical VAD, and a percutaneous 4-L Impella CP® device was implanted. Implantation was performed without complications via a right femoral approach (Figure), and the mean flow achieved of 3 L improved her clinical and hemodynamic profile (Table). Given the cardiogenic shock and dependence on a short-term VAD, she was prioritized on the national emergency heart transplant waiting list. The device was stopped after 10 days because it displayed a “high motor current” alarm. Because the patient showed renewed hemodynamic deterioration, the dysfunctional device was removed and a new one was implanted. After 4 days with this second device, the patient showed left homonymous hemianopia due to right occipital hemorrhage requiring urgent surgical drainage. It was decided to stop anticoagulation therapy and, thus, the VAD was removed while high-dose vasoactive support was maintained. After 2 weeks of proper neurological development and no hemorrhage and due to progressive hemodynamic deterioration, an intra-aortic balloon pump was implanted, without anticoagulation, and the patient was included on the emergency heart transplant list. The transplant was successfully performed 48hours later. Ten months after the heart transplant, the patient has minimal neurological sequelae that do not impede a normal life.
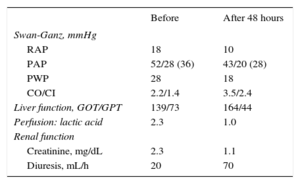
Hemodynamic and Analytical Data Before and After Impella CP® Implantation
| Before | After 48 hours | |
|---|---|---|
| Swan-Ganz, mmHg | ||
| RAP | 18 | 10 |
| PAP | 52/28 (36) | 43/20 (28) |
| PWP | 28 | 18 |
| CO/CI | 2.2/1.4 | 3.5/2.4 |
| Liver function, GOT/GPT | 139/73 | 164/44 |
| Perfusion: lactic acid | 2.3 | 1.0 |
| Renal function | ||
| Creatinine, mg/dL | 2.3 | 1.1 |
| Diuresis, mL/h | 20 | 70 |
CI, cardiac index; CO, cardiac output; PAP, pulmonary artery pressure; PWP, pulmonary wedge pressure; RAP, right atrial pressure.
The Impella device contains an axial pump that is placed across the aortic valve and continually draws blood from the left ventricle to the aorta. Recent trials support the use of the Impella 2.5 (percutaneous, 13 Fr) in high-risk coronary interventions but the flow provided (1.5-2.0 L) is insufficient for cardiogenic shock.1,2 The current report of the use of the Impella CP® (4 L) as a bridge to heart transplant is the first in Spain.
From this experience, we learned that percutaneous implantation is straightforward and can be performed in the catheterization laboratory in a mean time of 15min. For implantation, a 14-Fr introducer is required to pass the motor (12 Fr). This introducer is the peel-away type and fits a flexible 10-Fr sheath. This difference in diameter can allow bleeding around the sheath. In our patient, the risk of bleeding was controlled by adjusting the slipknot of the Proglide® around the 10-Fr sheath. Additionally, the Impella device moved between 2 cm and 4cm on 4 occasions. Relocalization was performed at the bedside with echocardiographic monitoring.
The Impella device can cause hemolysis, which can be reduced by optimization of the minimum revolutions required to maintain the desired flow; in our patient, the initial flow of 3.3 L with the maximum power (46 000rpm) was later decreased to between 2.8 L and 3.0 L (44 000rpm) to reduce hemolysis. This flow was sufficient to stabilize the patient (1.43 m2 body surface area) without overloading the right ventricle. If the flow had been insufficient and/or there had been refractory right ventricle failure, an extracorporeal membrane oxygenator would have been implanted as a VAD.
The first notable complication was the motor dysfunction that occurred on the tenth day after surgery. This is the first report of mechanical failure due to exceeding the approved duration of use (7 days). Currently, the mean waiting time for an urgent heart transplant is 8 days. Thus, this device offers hemodynamic support for a reasonable period of time while a patient is waiting on a national emergency transplant list; once the approved duration of use has elapsed, individualization of the strategy is required: replacement of the Impella Levitronix Centrimag or implantation of another longer-lasting VAD (Berlin Heart EXCOR®, HeartMate II, or HeartWare).
The second noteworthy complication was cerebral hemorrhage, which occurred when the patient was anticoagulated with sodium heparin with an activated clotting time of 168s (recommended time, 160-180s). The prevalence of hemorrhagic complications for axial flow devices is 24%; this type of flow can cause mechanical shear stress, a syndrome termed acquired von Willebrand syndrome.3 This condition affects small vessels; the most common complications being gastrointestinal bleeding. In our patient, we could not rule out this syndrome or the presence of pre-existing cerebral arteriovenous malformations.
In conclusion, the Impella CP® device can be an alternative to surgical VAD in selected patients with cardiogenic shock as a bridge to decision-making or heart transplant. This device is limited to between 7 and 10 days and is not without serious complications.