A high proportion of all cerebrovascular accidents (CVAs) are isquemic CVAs. In approximately 30% of patients, the cause is not determined and the CVA is considered cryptogenic. Atrial fibrillation (AF) is one of the most frequent causes of ischemic stroke, and so monitoring cardiac rhythm for long periods of time may help improve diagnosis. The use of implantable loop recorders that have algorithms for detecting AF may help to achieve this goal.1
We present a series of 14 consecutive patients referred from the neurology department between August 2009 and February 2011 with cryptogenic ischemic stroke and embolism. A Reveal® XT (Medtronic Inc., Minneapolis, United States) recorder was implanted in all patients to detect AF. Ten (71.4%) patients were men, and the mean (SD) age was 65.4 (10.9) years. Eleven (79%) had hypertension and 5 (36%) had diabetes mellitus. None of the patients had a history of AF. All patients had undergone a study that included daily electrocardiography, laboratory tests, brain computed tomography scan, external Holter monitoring, transthoracic and transesophageal electrocardiography, supra-aortic Doppler examination, and brain magnetic resonance angiography. Signs of mild atheromatosis without stenosis were detected in 4 patients in the supra-aortic trunk. The recorder was deployed in the left parasternal region in a subcutaneous pocket within a month of the episode. No complications were reported.
A follow-up visit was scheduled at 1 month and then every 3 months. Additional visits were scheduled in the event of symptoms or activation of the recorder's alarm. The devices in 10 patients (71%) recorded 24 episodes of AF that were not confirmed after manual review. Analysis of these episodes showed that they corresponded to the detection of noise, intermittent T-waves, or frequent extrasystoles.
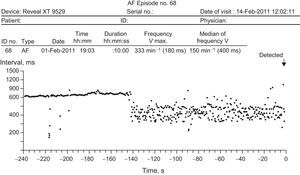
All patients attended the scheduled visits. The implantable loop recorder detected AF in 5 patients (35.7%) (Figure). The clinical characteristics of these patients and the number and duration of the episodes are shown in the Table. The mean time to detection after deployment was 5.8 months, and no patient reported symptoms related to the arrhythmia. In all cases, patients started treatment with oral antiplatelet therapy. The patients in whom no AF was detected during the study period had a mean age of 61 (10.5) years compared with 74 (5.8) years for those in whom AF was detected (P=.01), with a mean follow-up of 29 (5.9) months and a mean of 7.25 visits (range, 5-12).
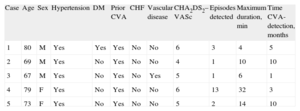
Characteristics of the 5 Patients in Whom Atrial Fibrillation Was Detected: CHA2DS2-VASc Score and the Characteristics of the Episodes
| Case | Age | Sex | Hypertension | DM | Prior CVA | CHF | Vascular disease | CHA2DS2–VASc | Episodes detected | Maximum duration, min | Time CVA-detection, months |
| 1 | 80 | M | Yes | Yes | Yes | No | No | 6 | 3 | 4 | 5 |
| 2 | 69 | M | Yes | No | Yes | No | No | 4 | 1 | 10 | 10 |
| 3 | 67 | M | Yes | No | Yes | No | Yes | 5 | 1 | 6 | 1 |
| 4 | 79 | F | Yes | No | Yes | No | No | 6 | 13 | 32 | 3 |
| 5 | 73 | F | Yes | No | Yes | No | No | 5 | 2 | 14 | 10 |
CHF, chronic heart failure or injection fraction <40%; CVA, cerebrovascular accident; DM, diabetes mellitus; F, female; M, male.
In our series, the implantable loop recorder allowed diagnosis of AF in 36% of the patients who had experienced cryptogenic CVA. In all of these patients, the arrhythmia was asymptomatic. The clinical guidelines and expert consensus statements recommend extensive cardiac monitoring in patients who have experienced a cryptogenic CVA and in those with suspicion of silent AF. The different methods of monitoring cardiac rhythm have their drawbacks, and their performance has been analyzed.2
The implantable loop recorder has been introduced only recently for detection of AF. Implantation is simple and performed under local anesthetic. The Reveal® XT recorder uses an AF algorithm based on detection of RR-interval irregularity, analyzed over 2-min periods. A study performed in patients with a high probability of AF showed high sensitivity (96.1%) and specificity (85.4%) for detection of the arrhythmia, although the presence of false positives was considered the main limitation of this technique.1
The performance of the implantable loop recorder with this indication is under investigation in 2 randomized studies, CRYPTONITE and CRISTAL AF. Both are prospective, multicenter studies in which an implantable loop recorder is deployed in patients who have recently had a stroke and whose full etiologic study is negative. To date, only 1 study of similar characteristics has been published.3 In that study, the Reveal Plus® 9526 monitor (Medtronic Inc., Minneapolis, United States) was implanted in 24 patients with the condition. After a mean follow-up of 14.5 months, AF had not been detected in any patients. The main limitation of the study is the device itself, which requires 32 consecutive beats at a frequency of more than 165 bpm to detect AF, which might not identify some AF episodes.
While awaiting the results of the ongoing studies, our study indicates that the implantable loop device with an algorithm for the detection of AF is a tool that should be considered in the etiologic study of cryptogenic CVA. However, given the lack of a control group in our study and the high prevalence of AF in patients with this profile, no causal relationship can be established, although the technique does allow the initiation of appropriate antiplatelet treatment.