Since robotic cardiac surgery was first performed at the end of the 1990s,1,2 the technique has gradually become more widespread. It has even been adopted by some leading centers in the United States as the approach of choice for mitral valve surgery. Although its adoption has been slower in Europe, both the case volume and the number of centers performing robotic surgery appear to have increased significantly in recent years.3
The objective of the present study was to report the outcomes of the first 120 patients who underwent robotic cardiac surgery in our hospital (from December 2019 to July 2022). The study was approved by the ethics committee of our center (HCB/2021/0248). The committee waived the need for informed consent from individual patients.
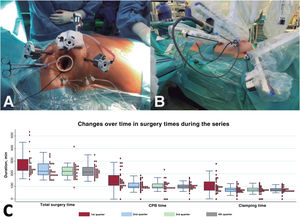
A single team of 2 surgeons (1 at the robot control console and 1 at the operating table) conducted all of the interventions with the DaVinci Xi platform (Intuitive Surgical, United States). Overall, 82% of the operations comprised mitral valve surgery (n = 98), and the most frequent procedure was mitral valve repair (n = 86) (figure 1A). The other procedures were atrial septal defect closure (n = 9), concomitant tricuspid surgery (n = 2), robotic dissection of the internal mammary artery to revascularize the anterior descending artery without cardiopulmonary bypass (CPB) (n = 7) (figure 1B), tumor excision (n = 3), and aortic valve replacement (n = 3). The characteristics of the cohort are shown in Table 1.
A: placement of the 4 robotic trocars and of the working port in the patient's right hemithorax, an arrangement used for intracavitary surgery. B: the 3 trocars placed in the left hemithorax to dissect the left internal mammary artery in coronary surgery patients. C: reduction in all surgery times (cardiopulmonary bypass [CPB], ischemia, and total surgery time), by quartile.
Description of the population and outcomes.
| Baseline characteristics | Total robotic cohort (n = 120) | Degenerative mitral valve robotic cohort (n = 78) | Degenerative mitral valve video-assisted cohort(n = 197) | P |
|---|---|---|---|---|
| Age, y | 58±15 | 59±14 | 59.87±12.7 | .47 |
| Male sex | 62% | 73% | 67.7% | .41 |
| Height, cm | 168±15 | 171±9 | 169.7±9.2 | .24 |
| Weight, kg | 74±19 | 75±15 | 73.2±13.6 | .26 |
| Hypertension | 29% | 33% | 40% | .29 |
| Dyslipidemia | 25% | 21% | 26.4% | .31 |
| Diabetes mellitus | 9% | 10% | 4.5% | .07 |
| Cerebrovascular disease | 5% | 3% | 4.5% | .44 |
| Chronic kidney disease | 6% | 6% | 2.5% | .12 |
| Creatinine, mg/dL | 0.95±0.3 | 0.99±0.3 | 0.95±0.32 | .49 |
| Ejection fraction, % | 60±8 | 61±7 | 61±6.5 | .92 |
| NYHA class > III-IV | 26.5% | 25% | 26.4% | .89 |
| EuroSCORE 2, % | 1.5 [0.7-1.7] | 0.9 [0.6-1.6] | 1.03 [0.69-1.88] | .09 |
| Localization of the mitral valve prolapse | ||||
| Anterior | — | 9% | 18 | .96 |
| Posterior | 65% | 124 | .70 | |
| Both flaps | 26% | 56 | .37 | |
| Intraoperative data | ||||
| CPB time, min | 105 [89-135] | 112 [92-140] | 118 [94-146] | .60 |
| Ischemia time, min | 74 [61-93] | 77 [67-100] | 89 [69-115] | .05 |
| Total surgery time, min | 225 [195-255] | 225 [197-259] | b | |
| Use of neochords | N/A | 64% | 46.7% | .009 |
| Resection | N/A | |||
| None | 64% | 54% | .19 | |
| Triangular | 33% | 35% | .85 | |
| Sliding plasty | 3% | 11% | .02 | |
| Annuloplasty | 96% | 100% | .02 | |
| Postoperative outcomes | ||||
| Mechanical ventilation, h | 7 [5-13] | 7 [4-21] | 7 [5-13] | .50 |
| Extubation in operating room | 60% | 59% | 61% | .76 |
| Extubation > 24 h | 12 (10) | 7 | 10 | |
| Length of stay | ||||
| Intensive care unit | 1 [1-2] | 1 [1-2] | 1 [1-2] | .29 |
| Hospital | 4 [4-6] | 4 [4-6] | 7 [6-10] | < .001 |
| Complications | ||||
| Vascular | 1 (0.8) | 1 (1) | 4 (2) | .67 |
| Stroke | 0 | 0 | 1 (0.5) | 1 |
| Renal failurea | 2 (1.6) | 2 (2.6) | 5 (2.5) | .99 |
| Permanent pacemaker | 0 | 0 | 5 (2.5) | .32 |
| Atrial fibrillation | 23 (19) | 17 (22.1) | 45 (22.8) | .85 |
| Transfusion | 24 (20) | 16 (20.5) | 37 (18.8) | .74 |
| Reoperation due to bleeding | 6 (5) | 4 (5) | 14 (7.1) | .55 |
| Aortic reclamping | 9 (7.5) | 7 (10) | 17 (8.6)b | |
| Respiratory | 14 (12) | 8 (10) | 1 (0.5) | .67 |
| Coronary lesion | 1 (0.8) | 1 (1) | .49 | |
CPB, cardiopulmonary bypass; NYHA, New York Heart Association functional class.
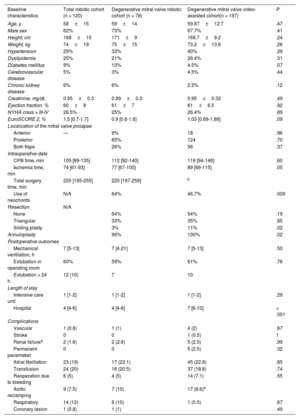
In the total cohort, the median [interquartile range] total surgery time was 225 [195-255] minutes while the median CPB and myocardial ischemia times were 105 [89-135] and 74 [61-93] minutes, respectively. All surgery times progressively decreased (figure) from program initiation, as follows (Q1 vs Q4): CPB time, 136 vs 98 minutes (P = .003); ischemia time, 92 vs 70 minutes (P = .009), and total surgery time, 240 vs 211 minutes (P = .02) (figure 1C).
Most patients (58%) were extubated in the operating room (median mechanical ventilation time of patients extubated in the operating room, 7 [5-13] hours). The median lengths of stay in the intensive care unit and in the hospital were 1 [1-2] and 4 [4-6] days, respectively. As seen with the surgery times, the length of hospital stay significantly decreased with experience (from Q1 to Q4): from 5 days to 4 days (P < .001). All times were comparable to those of a video-assisted mitral valve surgery cohort, except the clamping time, which was lower in the robotic surgery group.
The rate of repair in patients with mitral regurgitation was 100% (with slight or minor mitral regurgitation at discharge in 98.8%). All patients were discharged to home and none required reintervention during follow-up. Median postoperative length of hospital stay was much shorter in the robotic surgery group than in the video-assisted surgery group (4 vs 7 days, P < .001).
Despite representing the initial experience, which includes the entire learning curve, our results are satisfactory and in line with those of series published by highly experienced centers4–6 and with our own results for video-assisted mitral valve surgery. We believe that the learning curve was minimized by the extensive previous experience of the entire team with video-assisted surgery, which permitted a very high level of safety and quality from the outset, as shown by the absence of conversions to sternotomy, the superb rate of mitral valve repair, and the low incidence of postoperative complications.
In conclusion, robotic cardiac surgery in selected patients enables the performance of a wide variety of cardiac surgical interventions with excellent results and short postoperative hospital stay. Robotic surgery is currently the least invasive surgical option but involves a highly complex technique with a steep learning curve that can be minimized by extensive prior experience with video-assisted surgery.
FUNDINGWithout any external funding.
AUTHORS’ CONTRIBUTIONSE. Sandoval: manuscript drafting and editing of tables and figure. A. Muro: data collection and final revision of the manuscript. R. Navarro: final revision of the manuscript. A. García-Álvarez: final revision of the manuscript. M. Castellà: final revision of the manuscript. D. Pereda: conceptualization, figure editing, critical revision, and final revision of the manuscript.
CONFLICTS OF INTERESTNone of the authors have a conflict of interest to declare in relation to this study.