The SAFEHEART study was designed to analyze the situation of familial heterozygous hypercholesterolemia (FHH) and improve knowledge of this disease in Spain. Our objective was to determine the incidence rate of cardiovascular events, the estimated risk of developing an event and its modification, the use of lipid-lowering treatment, and the achievement of low-density lipoprotein cholesterol targets in patients with FHH.
MethodsSAFEHEART is a prospective, open, multicenter, nationwide cohort study, with long-term protocol-based follow-up in a population of individuals with molecularly-characterized FHH. We analyzed patients older than 18 years with complete follow-up.
ResultsWe included 2648 patients with FHH. The median follow-up was 6.6 (4.8-9.7) years. The overall incidence rate of cardiovascular events was 1.3 events/100 patient-years. After the follow-up, the 10-year estimated risk of developing a cardiovascular event was reduced from 1.6% to 1.3% (P <.001). In the last follow-up, 20.6% and 22.2% of the patients in primary and secondary prevention achieved low-density lipoprotein cholesterol values <100mg/dL and <70mg/dL, respectively.
ConclusionsThis study was performed in the largest population of patients with FHH in Spain. We identified the incidence rate of cardiovascular events, the estimated risk of developing a cardiovascular event and its modification, the achievement of low-density lipoprotein cholesterol targets, and the therapeutic management in this population. Although the cardiovascular risk of FHH is high, appropriate treatment reduces the likelihood of an event.
Clinical trial registration: http://www.clinicaltrials.gov. Identifier: NCT02693548.
Keywords
Heterozygous familial hypercholesterolemia (HeFH) is an autosomal codominant disorder with a prevalence of approximately 1 case per 300 people in the general population.1 It is the most common genetic disorder linked to premature atherosclerotic cardiovascular disease (ASCVD). Although lipid-lowering treatment has been shown to reduce coronary heart disease and overall mortality in patients with HeFH2—and has improved in recent years—most patients do not achieve optimal low-density lipoprotein cholesterol (LDL-C) levels3,4 and therefore continue to be at increased risk of premature ASCVD. Not all patients with HeFH, however, have the same CV risk. We recently published an equation for estimating individual CV risk in patients with HeFH based on 8 simple variables,5 including lipoprotein(a), which has proven to be an effective predictor of incident ASCVD in this setting.6
The latest European Society of Cardiology/European Atherosclerosis Society Guidelines for the Management of Dyslipidemias7 (ESC/EAS Guidelines) consider patients with HeFH to be at high or very high CV risk and recommend a target LDL-C level of less than 100mg/dL for patients without a history of CV disease and less than 70mg/dL for those with a history. They also recommend reducing baseline LDL-C levels by at least 50% in both cases. Little, however, is known about the use of lipid-lowering treatment or the attainment of LDL-C goals in Spain. National registries serve as an invaluable source of such data, which are essential for improving current health care models, educating physicians and patients, and setting priorities in treatment guidelines and health care policies.8 The SAFEHEART Spanish Familial Hypercholesterolemia Cohort Study was designed to collect nationwide data on FH in order to improve knowledge of this disease in Spain.
The aim of this study was to analyze data from the SAFEHEART registry to determine the incidence of CV events in patients with HeFH in Spain, estimate their risk of experiencing a cardiovascular event using the SAFEHEART Risk Equation (SAFEHEART-RE), assess changes to this risk, and analyze the use of lipid-lowering treatments and attainment of treatment goals on inclusion in the registry and at the most recent follow-up.
MethodsStudy design and populationSAFEHEART is a prospective, open-labeled, multicenter, national study with a protocol-based long-term follow-up program for patients with molecularly defined HeFH. It involves the participation of primary care physicians and specialists.9 The selection of families started in 2004. The study was approved by the ethics committee at Hospital Fundación Jiménez Díaz in Madrid and all participants gave their written informed consent.
Treatment goals were based on the ESC/EAS Guidelines,7 which were used to inform, educate, and train physicians participating in the study and to guide the inclusion of patients and families in the SAFEHEART registry. Patients were assigned to a given region (one of Spain's autonomous communities or cities) based on their current place of residence, not their place of birth.
Follow-upPatient follow-up was managed by the SAFEHEART coordinating center. Patients were contacted annually by telephone to complete a standardized questionnaire assessing relevant lifestyle changes, use of medication, and occurrence of CV events.9 All the patients included in the study had been contacted in the previous year. Complete follow-up was defined as contact with the patient in the 12 months prior to analysis and availability of all required data in the standardized annual follow-up form. The definitions of previous and incident ASCVD are described elsewhere.5 When the occurrence of a CV event was detected in the annual evaluation or reported at another time by a patient or relative, the SAFEHEART Events Committee analyzed the necessary medical-legal reports and classified the event using standardized criteria.
Clinical and laboratory variablesIn addition to the above-mentioned demographic and clinical variables, information was also included on age, classic CV risk factors, physical examination findings, and lipid-lowering treatment.9 Lipid and lipoprotein(a) levels were measured in venous blood samples at a single laboratory.9 Serum LDL-C levels were calculated using the Friedewald formula. DNA was isolated from whole blood samples using standard methods and the genetic diagnosis of HeFH was established as previously described.10 CV risk was estimated using the SAFEHEART-RE.5 The system used to classify intensity of lipid-lowering treatment has been described previously,5 but in this study protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors were also included in the maximal treatment category.
Statistical analysisThe statistical analysis was performed in SPSS (version 18.0). Quantitative data are expressed as median and interquartile range (IQR), except for the analyses stratified by region, where median values only are shown. Risk estimates and their modifications are expressed as mean following confirmation of their normal distribution. Qualitative data are shown as absolute numbers and percentages or as percentages only for the regional analyses. The incidence rate for CV events was calculated as a ratio in which the number of events observed was divided by time at risk for the event. Time at risk was calculated as the duration of follow-up for patients who did not experience an event plus time to occurrence in patients who did experience an event. Incidence rates were expressed as number of events per 100 patient-years. Only first occurrences of events, whether fatal or nonfatal, were included. The McNemar test was used to compare paired proportions, the Wilcoxon test to compare paired quantitative variables, and the binomial and Wilcoxon signed rank tests, respectively, to compare proportions and medians between regions and the country as a whole. Differences with a P value of less than .05 were considered to be statistically significant.
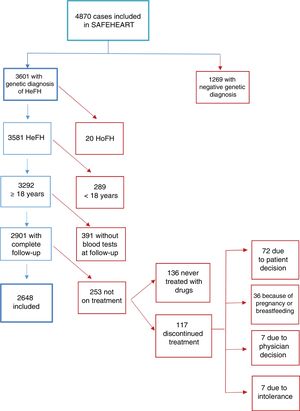
ResultsA total of 4870 individuals were recruited: 3601 with a genetic diagnosis of HeFH and 1269 unaffected relatives. After exclusion of patients without a complete follow-up and patients not receiving lipid-lowering treatment at the time of the most recent evaluation (last follow-up), 2648 patients with HeFH were included in the analysis (figure 1). Median follow-up time was 6.6 years (IQR, 4.8–9.7 years). The main characteristics of the cohort at entry in the registry and at the last follow-up are summarized in table 1 and table 2. The distribution of patients according to region of residence is shown in . The sample included patients from all regions except the autonomous communities of Cantabria, Murcia, and Navarre and the autonomous cities of Ceuta and Melilla.
Population characteristics (binary variables)
| Inclusion in registry | Most recent follow-up | |||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | Difference, % | P |
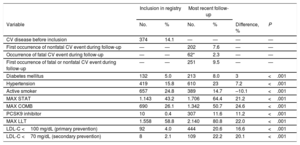
| CV disease before inclusion | 374 | 14.1 | — | — | — | — |
| First occurrence of nonfatal CV event during follow-up | — | — | 202 | 7.6 | — | — |
| Occurrence of fatal CV event during follow-up | — | — | 62* | 2.3 | — | — |
| First occurrence of fatal or nonfatal CV event during follow-up | — | — | 251 | 9.5 | — | — |
| Diabetes mellitus | 132 | 5.0 | 213 | 8.0 | 3 | <.001 |
| Hypertension | 419 | 15.8 | 610 | 23 | 7.2 | <.001 |
| Active smoker | 657 | 24.8 | 389 | 14.7 | –10.1 | <.001 |
| MAX STAT | 1.143 | 43.2 | 1.706 | 64.4 | 21.2 | <.001 |
| MAX COMB | 690 | 26.1 | 1.342 | 50.7 | 24.6 | <.001 |
| PCSK9 inhibitor | 10 | 0.4 | 307 | 11.6 | 11.2 | <.001 |
| MAX LLT | 1.558 | 58.8 | 2.140 | 80.8 | 22.0 | <.001 |
| LDL-C <100 mg/dL (primary prevention) | 92 | 4.0 | 444 | 20.6 | 16.6 | <.001 |
| LDL-C <70 mg/dL (secondary prevention) | 8 | 2.1 | 109 | 22.2 | 20.1 | <.001 |
CV, cardiovascular; LDL-C, low-density lipoprotein cholesterol; MAX COMB, maximal combined treatment; MAX LLT, maximal lipid-lowering treatment; MAX STAT, maximal statin treatment; PCSK9, proprotein convertase subtilisin/kexina type 9.
Maximal combined treatment with statins and ezetimibe 10mg/d.
Maximal treatment with statins (atorvastatin 40–80mg/d or rosuvastatin 20–40mg/d).
Maximal lipid-lowering treatment expected to reduce pretreatment LDL-C levels by at least 50%. Simvastatin 20, 40, or 80mg/d combined with ezetimibe 10mg/d; pravastatin 40mg/d combined with ezetimibe 10mg/d; fluvastatin 80mg/d combined with ezetimibe 10mg/d; atorvastatin 40 or 80mg/d combined or not with ezetimibe 10mg/d; atorvastatin 10 or 20mg/d combined with ezetimibe 10mg/d; rosuvastatin 20 or 40mg/d combined or not with ezetimibe 10mg/d; rosuvastatin 10mg/d combined with ezetimibe 10mg/d; pitavastatin 4mg/d combined with ezetimibe 10mg/d and PCSK9 inhibitor.
Of the 62 patients who experienced a fatal event, 13 (21%) had had a previous nonfatal event during follow-up.
Population characteristics (continuous variables)
| Variable | Inclusion in registry | Most recent follow-up | Difference | P |
|---|---|---|---|---|
| Age, y | 46 (36-58) | 54 (43-65) | 8 | <.001 |
| Body mass index | 26.1 (23.1-29.4) | 26.1 (23.4-29.0) | 0 | .9 |
| TC, mg/dL | 234.0 (203.0-275.0) | 196.0 (170.0-225.0) | –38.0 | <.001 |
| LDL-C, mg/dL | 163.0 (136.0-202.9) | 123.0 (100.0-147.0) | –40.0 | <.001 |
| Lipoprotein(a), mg/dL | 24.4 (9.3-58.5) | — | — | |
| Years under treatment with statins | — | 18.7 (12.8-26.5) | — | — |
| Years under treatment with ezetimibea | — | 10.9 (8.0-12.9) | — | — |
| SAFEHEART-RE 10,b % | 1.6 (0.7-3.7) | 1.3 (0.6-2.9) | –0.3 | <.001 |
LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
Values are expressed as median (interquartile range).
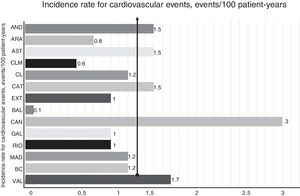
A total of 251 first fatal or nonfatal ASCVD events were recorded during follow-up; there were 202 nonfatal events and 62 fatal events. The nonfatal events were nonfatal acute coronary syndrome (83 patients, 3.1%), coronary revascularization (64 patients, 2.4%), nonfatal stroke (23 patients, 0.9%), peripheral revascularization (15 patients, 0.6%), and aortic valve replacement (17 patients, 0.6%). The fatal events were fatal acute coronary syndrome (16 patients, 0.6%), fatal stroke (8 patients, 0.3%), and other CV death, including sudden death (38 patients, 1.4%). Thirteen (21%) of the 62 patients with a fatal event had had a previous CV event during follow-up. All patients who experienced an event (and/or their relatives) were contacted. Of the 251 patients who experienced a fatal or nonfatal event during follow-up, 114 (45.4%) had had a CV event before inclusion in the registry. The overall incidence of CV events was 1.3 events per 100 patient-years. The breakdown by region is shown in figure 2. The proportion of patients on maximal lipid-lowering treatment increased from 58.8% at entry to 80.8% at the last follow-up (P<.001), while that of patients on PCSK9 inhibitors increased from 0.4% to 11.6% (P<.001). In the group of patients excluded because they were not on lipid-lowering treatment at the last follow-up (n=253), there were 5 first nonfatal events and 6 first fatal events (preceded by a nonfatal event in 2 cases). In the group of patients excluded because of nonavailability of LDL-C results at the last follow-up (n=391), there were 10 first nonfatal events and 12 first fatal events, 1 of which was preceded by a nonfatal event.
Incidence of cardiovascular events according to place of residence. AND, Andalusia; ARA, Aragon; AST, Asturias; BAL, Balearic Islands; BC, Basque Country; CAN, Canaries; CAT, Catalonia; CL, Castilla-León; CLM, Castilla-La Mancha; EXT, Extremadura; GAL, Galicia; MAD, Madrid; RIO, La Rioja; VAL, Valencia.
At entry, 4.0% of patients in primary prevention and 2.1% of those in secondary prevention met their respective LDL-C goals of less than 100mg/dL and less than 70mg/dL. The corresponding rates for patients meeting their goals at the last follow-up were 20.6% and 22.2% (P<0.001). The proportion of active smokers fell from 24.8% at entry to 14.7% at the last follow-up (P <.001). There were no significant changes in body mass index (BMI) during this period.
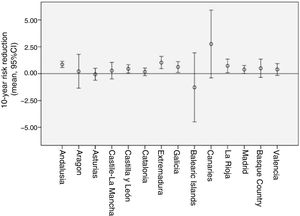
Mean changes in CV risk from entry to last follow-up according to the SAFEHEART-RE and stratified by place of residence are shown in figure 3 together with the corresponding 95% confidence intervals. The results for the other variables stratified by place of residence are shown in . In total, 37.5% of the patients were treated in primary care.
DiscussionWe have described, for the first time, the incidence of CV events in patients with HeFH in Spain and have provided additional data on CV risk, use of lipid-lowering treatment, and attainment of treatment goals at a country and regional level. The overall incidence rate detected for CV events was 1.3 events per 100 patient-years.
Our findings show that intensification of lipid-lowering treatment in patients with HeFH improved LDL-C levels over the follow-up period of 6 years. During this time, the proportion of patients on maximal lipid-lowering treatment rose from 58.8% to 80.8%. Although 80.8% of patients were on maximal lipid-lowering treatment and 11.6% were on PCSK9 inhibitors at the last follow-up evaluation, just 20.6% of patients in primary prevention and 22.2% of those in secondary prevention had optimal LDL-C levels (<100mg/dL and <70mg/dL, respectively. A number of points should be made regarding LDL-C goals: a) the proportion of patients with optimal LDL-C levels has doubled since the last analysis of patients from the SAFEHEART registry3; b) attainment of goals was more common in secondary than in primary prevention patients, probably because of the recent incorporation of PCSK9 inhibitors for patients at increased risk; and c) the proportion of patients failing to meet their LDL-C goals remains high, possibly in part because patients with FH have very high initial LDL-C levels; this highlights the importance of recommending potent statins and combination therapy with ezetimibe and PCSK9 inhibitors in patients with HeFH.11 The findings of this study show the persistence of a considerable gap between treatment goals and practice, confirming that there is still much room for improvement. They also, however, show just how difficult it is for patients with HeFH to achieve optimal LDL-C levels, despite treatment with the best lipid-lowering treatments available. It should be noted that the statistical power for comparing results across the different regions of Spain was limited by the characteristics of the registry. A recent study on the incidence of CVD in patients with FH in Catalonia reported a similar incidence rate to that observed in the SAFEHEART registry, despite using a different methodology.12
The improvement in CV risk observed over the follow-up period is probably due to the significant reduction in LDL-C levels. Although the prevalence of clinical CVD and hypertension has increased, there has been a significant reduction in tobacco use among patients with HeFH. Mean BMI was 26.1, which is lower than the BMI for the average Spanish adult: 26.7 (27.2 for men and 26.1 for women). In addition, mean BMI did not change during follow-up, contrasting with the situation for Spanish adults in general, who tend to have increasing BMI as they age.13 In part, patients with HeFH probably maintain a better BMI because they are more aware of the importance of CVD prevention and healthy lifestyle habits.14
Before the emergence of new tools for assessing CV risk in patients with FH, baseline LDL-C levels (levels before initiation of lipid-lowering treatment) were used to identify severe phenotypes.15,16 The SAFEHEART-RE, which is based on prospective data, provides a more accurate tool for defining risk and guiding decisions on the most appropriate treatment strategies for patients with HeFH.5
A final note of interest is that 37.5% of patients with HeFH are treated in primary care, although rates varied considerably across regions. One example is Castile-León, which has a regional FH detection program that involves the participation of primary care physicians.17
We are aware of the strengths and limitations of the present study. This is the largest longitudinal study to analyze real-life treatment practices at different levels of the health care system in patients with molecularly defined HeFH. Although SAFEHEART is a national registry, it does not include patients from all the autonomous communities in Spain. Our results show the need for a prospective registry to evaluate trends in CV healthcare provision in the setting of HeFH.
Practical implicationsThe lack of FH screening programs is a barrier to the effective prevention of premature ASCVD and has a negative impact on the quality of life and socioeconomic situation of families with HeFH. Early detection and treatment of HeFH remains a health care challenge and constitutes an unmet medical need. A highly efficient national screening program for FH will help prevent CV morbidity and mortality in families with HeFH, improve overall health care delivery, and overcome regional healthcare disparities.18
ConclusionsThis study is the first to show the incidence of CV events in a large population of patients with HeFH and to analyze associated risk and changes in risk, attainment of treatment goals, and treatment strategies employed in this population. Although the risk is high, adequate treatment considerably reduces the likelihood of a CV event. Efforts should thus focus on achieving adequate LDL-C control and improving risk factors in patients with HeFH.
Ackn*owledgmentsWe would like to thank the staff at Fundación Hipercolesterolemia Familiar for their invaluable help with the recruitment and follow-up of patients in the registry. We are also very grateful to all the families who collaborated in this study.
FundingThis study was funded by the Fundación Hipercolesterolemia Familiar; grant G03/181 and FIS PI12/01289 of the Instituto de Salud Carlos III (ISCIII) and grant 08-2008 from the Centro Nacional de Investigación Cardiovascular (CNIC).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
- –
HeFH is a common disorder frequently associated with premature ASCVD.
- –
Numerous studies have shown that lipid-lowering treatment can reduce mortality in FH.
- –
National registries are a valuable source of key information. The SAFEHEART study was designed to improve knowledge about HF in Spain.
- –
This study is the first to show the risk of CV events in a large population of patients with HeFH and to analyze changes to this risk, achievement of treatment goals, and treatment strategies.
- –
Although CV risk is high in patients with HeFH, adequate treatment considerably reduces the likelihood of a CV event.
- –
It is important to focus efforts on achieving adequate control of LDL-C in patients with HeFH.
Rocío Aguado, Begoña Perez-Corral (Hospital Universitario de León); Fátima Almagro (Hospital Donostia, San Sebastián); Rodrigo Alonso, Raquel Arroyo, Nelva Mata, Pedro Mata, Leopoldo Pérez de Isla, Adriana Saltijeral (Fundación Hipercolesterolemia Familiar); Francisco Arrieta (Hospital Ramón y Cajal, Madrid); Lina Badimón, Teresa Padró (Instituto Ciencias Cardiovasculares, IIB-Sant Pau, Barcelona); Miguel Ángel Barba (Hospital Universitario, Albacete); Ángel Brea, Daniel Mosquera, Marta Casañas (Hospital San Pedro, Logroño); Julio Carbayo (Clínica Virgen del Rosario, Albacete); Jose María Cepeda (Hospital de Vega Baja, Orihuela); Raimundo de Andrés (Fundación Jiménez Díaz, Madrid); José L. Díaz (Hospital Abente y Lago, A Coruña); Gonzalo Díaz-Soto (Hospital Clínico, Valladolid); Marta Diéguez, María Riestra (Hospital de Cabueñes, Gijón); Francisco Fuentes, José López-Miranda (Hospital Reina Sofía, Córdoba); Jesús Galiana, M. Dolores Mañas (Hospital de Ciudad Real); Jesús García-Cruces (Hospital Río Hortega, Valladolid); Juan Antonio Garrido (Hospital de Ferrol; Luis Irigoyen (Hospital Clínico Universitario Lozano Blesa, Zaragoza); Pedro L. Martínez (Hospital La Paz, Madrid); Ceferino Martínez-Faedo, Lorena Suárez (Hospital Central de Asturias, Oviedo); Marta Mauri, Rosa M. Borrallo (Hospital de Terrassa, Barcelona); Juan Diego Mediavilla, Fernando Jaén, Pablo González (Hospital Virgen de las Nieves, Granada); Alfredo Michán, Patricia Rubio (Hospital Jerez de la Frontera); Pablo Miramontes (Hospital Clínico Universitario, Salamanca); Juan L. Morera (Hospital Vital Álvarez Buylla, Mieres); Ovidio Muñiz, Aurora González (Hospital Virgen del Rocío, Sevilla); Francisca Pereyra (Hospital Universitario Nta. Sra. Candelaria, Tenerife); Leire Pérez (Hospital Universitario de Álava); Mar Piedecausa, José Pastor (Hospital Universitario de Elche); José Miguel Pinilla (Centro de Salud San Miguel de Salinas, Alicante); Xavier Pintó (Hospital de Bellvitge, Barcelona); Manuel J. Romero (Hospital Infanta Elena, Huelva); Enrique Ruiz, M. Pilar Álvarez (Hospital Universitario, Burgos); Pedro Sáenz (Hospital de Mérida); Juan F. Sánchez (Hospital San Pedro de Alcántara, Cáceres); Consuelo Sanz (Centro de Salud de Lerma-Zael); Jose I. Vidal, Rosa Argüeso (Hospital Universitario, Lugo), y Daniel Zambón (Hospital Clínic, Barcelona).
Full list available at: https://www.colesterolfamiliar.org/estudio-safeheart/centros-participantes-en-el-estudio/.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2019.10.028