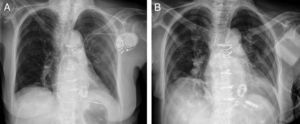
Patients with mechanical prostheses require continuous anticoagulation with vitamin K antagonists, which frequently perform erratically in response to efforts to maintain therapeutic levels of anticoagulation. Their use considerably increases the risk of hematomas involving the generator pocket created for cardiac implantable electronic devices.
We present the case of an 80-year-old woman with long-standing rheumatic valve disease and a mechanical mitral valve prosthesis, who underwent subcutaneous implantation, in the left pectoral region, of a pacemaker system with an active fixation lead in the apex of the right ventricle, to treat atrial fibrillation with poor control of the ventricular response (Figure A). Several days after implantation, the patient developed a hematoma under tension and an opening in the pocket. She had an international normalized ratio (INR) of 5. The entire system was extracted and a drain was left in place for several days (Figure B). When the drain was removed and the wound closed, the patient developed a new hematoma under tension, which again required surgical evacuation and placement of a vacuum system. To avoid the same complication with the placement of a new pacing system in the right side, a Micra™ leadless transcatheter pacemaker (Medtronic) was implanted via the femoral vein into the right ventricular apical septum without incident (Figure B). Subsequent evaluation showed adequate detection and pacing, without incidents. The implantation was performed under uninterrupted acenocoumarol therapy, with the patient's INR at 2.8. Despite the large-gauge introducer sheath required for implantation via the femoral vein (27 F) and the maintenance of anticoagulation therapy, hemostasis was achieved with a subcutaneous figure-of-eight suture. The results were excellent, with no hematoma or other complications. Leadless pacemakers use a new technology developed to avoid complications related to both the surgical wound and the generator pocket, as well as the transvenous endocardial leads.