Hypertrophic cardiomyopathy is the most common type of cardiomyopathy, with a prevalence of 0.2% in the adult population. The diagnosis is based on finding an increased myocardial thickness of ≥ 15 mm that is unexplained by abnormal loading conditions.1
Dynamic left ventricular outflow tract obstruction (LVOTO), defined by a peak Doppler gradient ≥ 30 mmHg, is a common condition that is found at presentation in a third of patients and is provocable in another third. This phenomenon is produced due to the combined action of septal hypertrophy and anterior systolic motion (ASM) of the mitral valve, which usually has morphological abnormalities. LVOTO increases morbidity and mortality, as it is associated with heart failure, angina, syncope, and sudden death.1,2
For patients with significant obstruction and limiting symptoms despite pharmacological treatment, invasive treatment, either surgical or alcohol septal ablation, is the therapeutic option of choice. The classic surgical approach is transaortic myectomy, or Morrow technique, whose results in terms of gradient resolution and symptomatic improvement have been proven extensively. However, the technique is not free from complications, mainly atrioventricular block, ventricular septal defects, and the onset of aortic regurgitation.2
Recently, new surgical techniques have been developed that combine myectomy with mitral interventions. Dulguerov et al.3 described good outcomes using a combined intervention that included transaortic and transmitral myectomy, elongation of the anterior mitral leaflet using a pericardial patch, partial resection of the posterior mitral leaflet, and annuloplasty. Other groups such as that of Ferrazzi et al.4 reported that performing shallow myectomies and resection of secondary chordae in patients with LVOTO and mild hypertrophy was associated with clinical and hemodynamic improvement. However, teams such as the Mayo Clinic team have advocated the exclusive practice of transaortic myectomy as a better technique, as in their series, the presence of ASM, residual obstructive gradient, or significant mitral regurgitation was limited to 1.7% of patients with an adequate myectomy.5
We present the case of a 56-year-old man with a diagnosis of hypertrophic obstructive cardiomyopathy, with a mutation identified in the TNNT2 gene (p.Asn271Ile). This mutation has been published and identified in more than 15 families in our center, and cosegregation has been demonstrated.6
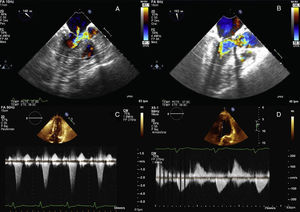
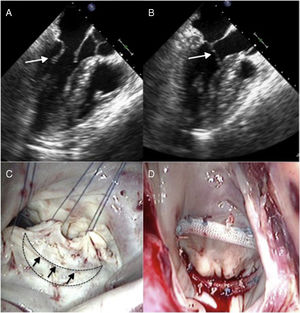
Cardiac magnetic resonance showed hypertrophy of the anterior and anteroseptal basal segments (maximum thickness, 17mm) without late enhancement. On stress echocardiography, an LVOTO gradient was observed with exercise (increasing from 17 to 120mmHg) with ASM and moderate mitral regurgitation (Figure 1A and C). Treatment with beta-blockers was started and the patient remained stable (New York Heart Association functional class I). The patient progressed to have moderate exertional dyspnea and chest pain compatible with angina, with no lesions seen on coronary angiography. On resting echocardiography, LVOTO was observed with the Valsalva maneuver (increasing from 15 to 50mmHg), with moderate mitral regurgitation caused by ASM of the posterior mitral leaflet, which was markedly elongated (Figure 2A and B and ).
A: transesophageal echocardiogram before surgery; color Doppler of the outflow tract showing obstruction; the arrow indicates mitral regurgitation. B: transesophageal echocardiogram after surgery; color Doppler of the outflow tract showing resolution of the mitral regurgitation and of the obstruction. C: stress echocardiogram before surgery; continuous Doppler of the outflow tract showing a significant gradient. D: stress echocardiogram after surgery; continuous Doppler of the outflow tract showing resolution of the gradient.
A and B: transesophageal echocardiogram; the arrow indicates the elongated posterior leaflet, with systolic anterior movement. C: surgical view; the discontinuous line indicates the semilunar resection of the posterior leaflet, and the arrows indicate where the edges will be sutured. D: surgical result; reduction in posterior leaflet area.
Disopyramide and bisoprolol were added, but, despite maximum tolerated doses, the patient remained symptomatic, and it was decided to perform an invasive intervention. In the absence of severe septal hypertrophy and given that the main mechanism of the obstruction appeared to be ASM of the posterior leaflet, it was decided to attempt mitral repair without myectomy.
The intervention was performed with a longitudinal semilunar resection of the posterior leaflet, suturing of the resection margins, and annuloplasty with a 32-mm ring (Figure 2C and D). This technique is used regularly in mitral repairs in patients with isolated mitral valve disease. The perioperative transesophageal echocardiogram and transthoracic echocardiogram performed immediately after surgery showed no ASM or resting or provocable LVOTO (Figure 1B and ).
After the repair, disopyramide was stopped and the patient progressed well and recovered to New York Heart Association functional class I. On postoperative stress echocardiography, neither ASM nor LVOTO were seen (Figure 1D).
In summary, in patients with hypertrophic cardiomyopathy with mild hypertrophy and a clear abnormality of the valvular apparatus, mitral repair may resolve the LVOTO and avoid the inherent risks of a myectomy.
FUNDINGJ.J. Cuenca-Castillo, J. Peteiro-Vázquez, and R. Barriales-Villa participate in Centro de Investigación Biomédica en Red (CIBERCV), Instituto de Salud Carlos III, CB16/11/00425.