Cancer therapy-induced cardiotoxicity (CTiCT), in the form of heart failure with reduced ejection fraction (HFrEF), is increasingly recognized. The effect of newer therapies such as sacubitril/valsartan in this form of HFrEF has not been established. In fact, a history of chemotherapy-related heart failure less than 12 months previously was an exclusion criterion for the PARADIGM-HF trial.1 Given the clinical benefits of sacubitril/valsartan, this therapy is starting to be used in real-life patients with CTiCT, but its effectiveness in this setting has not been tested.
The aim of this study was to evaluate the effects of sacubitril/valsartan on left ventricular ejection fraction (LVEF) and reverse remodelling parameters, assessed by cardiac magnetic resonance (CMR), in patients with cancer therapy-induced cardiomyopathy.
We present data from 10 consecutive patients with CTiCT who underwent a comprehensive multiparametric CMR before and> 3 months after sacubitril/valsartan initiation in our cardio-oncology clinic. CMR examinations were conducted with a Philips 1.5-Tesla Achieva whole-body scanner (Philips Healthcare) equipped with a 16-element phase-array cardiac coil and fully installed and managed by the Cardiology Department at Salamanca University Hospital.2 At all-time points, the imaging protocol included a standard segmented cine steady-state free-precession sequence to provide high-quality anatomic references, a multi-echo gradient-spin-echo sequence, and a modified look locker inversion (MOLLI) recovery with-5(3)3 acquisition scheme to provide T2 and native T1 relaxation times, respectively. CMR images were analyzed using dedicated software (MR Extended Work Space 2.5, Philips Healthcare; and QMassMR 7.6, Medis) at the Salamanca University Hospital with supervision from the CNIC (National Center for Cardiovascular Research) and blinded to time-point allocation and patient identification.
We conducted a complete medical history, physical examination, electrocardiogram, and blood sample extraction including N-terminal pro-brain natriuretic peptide (NT-proBNP) and renal function for each participant at baseline and at follow-up.
Data are presented as mean and standard deviation (SD) for normally distributed quantitative variables and as median and interquartile range [IQR] for non-normally distributed variables. For categorical variables, data are expressed as frequencies and percentages. The paired sample Student t test and Wilcoxon signed rank test were used for comparisons. All reported P values are 2-sided. Statistical analyses were performed with SPSS, version 23.
The median age was 73 [IQR 65-78] years; 60% were male and 90% had at least 1 cardiovascular risk factor (60% dyslipidemia, 50% hypertension, and 40% diabetes). Two patients (20%) were diagnosed with previous atrial fibrillation. Six of these patients had lymphoma, 2 had breast cancer, 1 had myeloma and 1 had lung cancer. The anticancer agents to which patients had been exposed were wide-ranging and had a variety of toxic mechanisms; 80% of the population received anthracyclines, 80% alkylating agents, 60% antimicrotubule agents, 60% rituximab, 20% antimetabolites, 20% PD-1 inhibitors, and 10% trastuzumab, anti-VGEF antibodies, lenalidomide, or pomalidomide. Combinations of therapy were used in 100% of the patients. No patients had received thoracic radiotherapy.
The median time from anticancer therapy to HFrEF was 31 [IQR 9-113] months. The median time from HFrEF to sacubitril/valsartan initiation was 11 [IQR 2-24] months. All patients were treated with triple HFrEF therapy before initiating sacubitril/valsartan. Functional class by then was New York Heart Association (NYHA) II in 6 patients, III in 3, and IV in 1. Most patients (70%) initiated sacubitril/valsartan at the lowest dose of 24/26mg. A total of 50% of patients achieved the dose of 49/51mg and 10% the target dose of 97/103mg at the end of follow-up. No patient in our study discontinued sacubitril/valsartan.
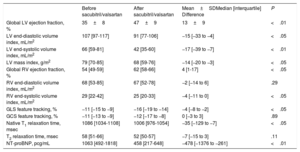
CMR data are presented in table 1. In summary, from baseline to follow-up CMR, there was a significant reduction in left ventricular (LV) volumes and a significant improvement in LVEF. A parallel significant reduction in NT-proBNP levels was also observed. When echocardiographic parameters and NT-proBNP levels were evaluated according to the sacubitril/valsartan dose received, there were improvements regardless of whether the patient was receiving a low dose or medium/high doses. Finally, at the time of follow-up CMR, a significant improvement in NYHA functional class was observed with 40% of patients in NYHA I, and 60% in NYHA II. No clinical adverse events were observed.
Patients’ CMR functional and structural parameters before and after sacubitril/valsartan treatment
| Before sacubitril/valsartan | After sacubitril/valsartan | Mean±SDMedian [interquartile] Difference | P | |
|---|---|---|---|---|
| Global LV ejection fraction, % | 35±8 | 47±9 | 13±9 | <.01 |
| LV end-diastolic volume index, mL/m2 | 107 [97-117] | 91 [77-106] | −15 [−33 to −4] | <.05 |
| LV end-systolic volume index, mL/m2 | 66 [59-81] | 42 [35-60] | −17 [−39 to −7] | <.01 |
| LV mass index, g/m2 | 79 [70-85] | 68 [59-76] | −14 [−20 to −3] | <.05 |
| Global RV ejection fraction, % | 54 [49-59] | 62 [58-66] | 4 [1-17] | <.05 |
| RV end-diastolic volume index, mL/m2 | 68 [53-85] | 67 [52-78] | −2 [−14 to 6] | .29 |
| RV end-systolic volume index, mL/m2 | 29 [22-42] | 25 [20-33] | −4 [−11 to 0] | <.05 |
| GLS feature tracking, % | −11 [−15 to −9] | −16 [−19 to −14] | −4 [−8 to −2] | <.05 |
| GCS feature tracking, % | −11 [−13 to −9] | −12 [−17 to −8] | 0 [−3 to 3] | .89 |
| Native T1 relaxation time, msec | 1086 [1034-1108] | 1006 [976-1054] | −35 [−129 to −7] | <.05 |
| T2 relaxation time, msec | 58 [51-66] | 52 [50-57] | −7 [−15 to 3] | .11 |
| NT-proBNP, pcg/mL | 1063 [492-1818] | 458 [217-648] | −478 [−1376 to −261] | <.01 |
GCS, global circumferential strain; GLS, global longitudinal strain; LV, left ventricle; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RV, right ventricle.
Values are expressed as mean±standard deviation or median [interquartile range].
To the best of our knowledge, this is the first study to report changes in LV function and anatomy in patients with CTiCT and HFrEF after sacubitril/valsartan treatment assessed with CMR.
Although cancer therapies have dramatically improved cancer-free survival, they have been accompanied by increasing CTiCT. LV dysfunction is potentially reversible, but crucially depended on the time to treatment with renin-angiotensin-aldosterone inhibitors and beta-blockers. We hypothesize that the observed benefit is likely related to the incremental benefits of neprilysin inhibition, which may further counteract the detrimental effects of the renin-angiotensin-aldosterone system and sympathetic nervous system activation. Thus, after our initial observations, we can speculate that sacubitril/valsartan could be the preferred approach for inhibiting the renin-angiotensin system in cancer survivors with CTiCT and LVEF reduction.
We have only identified in the literature a report of 2 cases of anthracycline-induced cardiomyopathy survivors successfully managed with sacubitril/valsartan,3 and in the oncology setting, only one similar study addressing improvements in echocardiographic LVEF and reverse remodelling with sacubitril/valsartan treatment.4
In conclusion, we present imaging and clinical data pertaining to CTiCT patients, before and after treatment with sacubitril/valsartan. After the treatment, we found improvements in CMR functional and structural parameters, NT-proBNP, and symptomatic status. Although the demonstration of a beneficial effect of sacubitril/valsartan is intriguing, the conclusions of this small observational study remain merely speculative. Thus, further observational studies with a larger number of patients are required to confirm our initial results.
FUNDINGThis study was supported by the Spanish Cardiovascular Network (CIBERCV) and was funded by an Excellence Project Integrated into the IIS grant (PIE14/00066) from the Institute of Health Carlos III, Ministry of Science, Innovation and Universities, Government of Spain; and with European FEDER funds.