Telemedicine enables the remote provision of medical care through information and communication technologies, facilitating data transmission, patient participation, promotion of heart-healthy habits, diagnosis, early detection of acute decompensation, and monitoring and follow-up of cardiovascular diseases. Wearable devices have multiple clinical applications, ranging from arrhythmia detection to remote monitoring of chronic diseases and risk factors. Integrating these technologies safely and effectively into routine clinical practice will require a multidisciplinary approach. Technological advances and data management will increase telemonitoring strategies, which will allow greater accessibility and equity, as well as more efficient and accurate patient care. However, there are still unresolved issues, such as identifying the most appropriate technological infrastructure, integrating these data into medical records, and addressing the digital divide, which can hamper patients’ adoption of remote care. This article provides an updated overview of digital tools for a more comprehensive approach to atrial fibrillation, heart failure, risk factors, and treatment adherence.
Keywords
Cardiovascular disease is one of the leading causes of morbidity and mortality worldwide and a paradigm of chronic diseases. Conventional in-person follow-up for these conditions has several drawbacks, such as geographical barriers, logistic challenges, accessibility limitations, and cost and time expenditure, and imposes a significant burden on the health care system. The situation is worsened by progressive population aging and the rising prevalence of cardiovascular risk factors and diseases.
Furthermore, despite the availability of effective evidence-based therapies for these conditions, the long-term benefits in real-life practice do not match those achieved in clinical trials, possibly due to a lack of adherence to the treatments prescribed.
For some time now, we have been immersed in a digital revolution. The recent COVID-19 pandemic accelerated the adoption of telemedicine and eHealth strategies, as health care professionals were compelled to reduce in-person visits. Telemedicine, a method for delivering medical services through the use of information and communication technologies to provide remote care, enables healthcare professionals to assess, diagnose, and treat patients without the need for an in-person encounter.
Health care based on telemedicine and portable devices that provide all the necessary information offer an opportunity to redesign and improve the care of cardiovascular disease patients. However, as they are medical devices, these tools should be regulated by competent authorities.
This review offers an up-to-date overview of currently available digital tools to provide a more comprehensive approach to arrhythmias, heart failure, and cardiovascular risk factors, as well as personal recommendations by the authors based on current evidence.
TOOLS FOR DETECTING AND MONITORING ARRHYTHMIASDigital tools for arrhythmia detectionTimely detection of arrhythmias enables prompt implementation of appropriate invasive and pharmacological interventions, and in some cases, leads to reductions in morbidity and mortality.1 In this regard, 12-lead electrocardiography (ECG) remains the standard for diagnosing arrhythmias. Nonetheless, access to an ECG study may be limited and evaluations with this technique can prove ineffective for diagnosing paroxysmal arrhythmias if the test is performed between episodes. Other conventional screening methods such as ambulatory Holter monitoring may be limited by restricted availability and a relatively short monitoring time. Implantable event recorders overcome this obstacle, but their high initial cost limits their use.2 Driven by the COVID-19 pandemic, the development of digital devices for detecting and monitoring cardiac arrhythmias has intensified. Although many professionals have already incorporated these devices into their clinical practice, there are concerns regarding their accuracy and usefulness, as well as their suitability for integration into treatment algorithms.3 The European Heart Rhythm Association recently published a document to guide the use of these technologies.3 While they offer multiple advantages, clinicians must become familiar with the acquisition and interpretation of the data they provide, as well as their limitations.
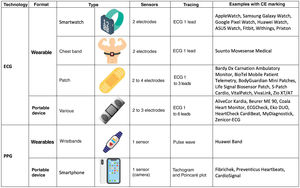
According to their technological basis, the devices fall into 2 main categories: those that use electrocardiographic signals and those that rely on non-electrocardiographic signals, with photoplethysmography being a notable example of the latter. These technologies can be incorporated into wearables in the form of clothing or accessories, such as smartwatches, patches, and textiles, or into portables, such as smartphones and dedicated devices (figure 1).
The diagnostic accuracy of these devices depends on the incorporated algorithm, the population using them, the conditions under which the recording is carried out, and the professional interpretation of the tracings. Recommendations for their use should be based on the frequency of symptoms, the desired monitoring time, the center's infrastructure, and the patients’ digital proficiency and personal preferences.
Devices based on electrocardiographic signalsThese options use electrodes to obtain electrocardiographic tracings, which a trained health care professional can directly analyze and interpret to diagnose cardiac arrhythmias.
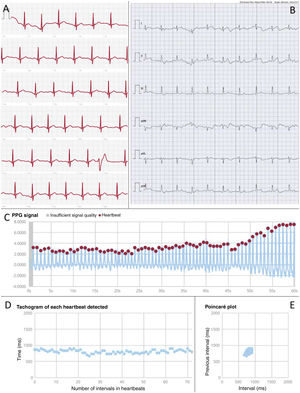
There are numerous commercially available devices for this purpose, many of which have been validated in clinical studies. They are mainly designed to detect atrial fibrillation (AF), and some have CE marking certification. Among wearables, smartwatches are particularly popular. These contain electrodes built into the watch case or crown to generate single-lead ECG tracings that can be reviewed on the device's screen or stored in mobile applications (figure 2A). Another option is patches, which are simple-to-use water-resistant adhesive devices that have shown a high diagnostic yield. Their main limitations are related to battery life, the adhesive component (although some are reusable), and chest bands. In addition, there are numerous portable devices, which mainly provide single-lead tracings (usually lead I), although some allow for up to 6 leads (figure 2B).
Examples of heart rhythm records provided by digital devices. A: single-lead ECG recording obtained using an Apple Watch smartwatch (Apple, United States) in a patient with sinus rhythm and a ventricular extrasystole. B: 6-lead ECG recording obtained using the Kardia Mobile 6L dedicated device (AliveCor, United States) in a patient with sinus rhythm. C: photoplethysmographic signal obtained using a smartphone and processed with the FibriCheck application (Corda Campus, Belgium) showing the intensity of the photoplethysmographic signal for each heartbeat over 1minute in a patient with sinus rhythm. D: tachogram derived from the previous photoplethysmographic signal showing the duration of the R-R interval for each heartbeat. E: Poincaré plot obtained from the previous photoplethysmographic signal showing the randomness of the cardiac rhythm by representing the R-R interval of each beat relative to the previous beat. F: photoplethysmographic signal, tachogram, and Poincaré plot obtained with the same application in a patient with atrial fibrillation. Note the irregular signal intensity and beat intervals, as well as the dispersion of points on the Poincaré plot, indicating the random nature of the rhythm. PPG, photoplethysmography.
Photoplethysmography stands out among nonelectrocardiographic technologies. This optical technique uses a light source and a sensor capable of detecting variations in blood volume on the skin surface through changes in the intensity of reflected light. The technique enables heart rate monitoring through the peripheral pulse. Information from the pulse wave is analyzed by integrated algorithms that can detect abnormalities. When AF is suspected, the patient is issued an alert. However, a subsequent electrocardiographic recording is needed to confirm the finding and establish a definite diagnosis of arrhythmia. Some devices allow for the analysis of raw data (figure 2C) and provide visual depictions using tachograms (figure 2D), which display the heart rate for each beat, and Poincaré plots (figure 2E), which show the R-R interval of each beat compared with the preceding beat. Some graphical representations can offer insights into the presence of specific arrhythmias other than AF.4
Photoplethysmography is commonly used in medical devices that measure heart rate and oxygen saturation but can also be incorporated into all types of wearables (wristbands, smartwatches, chest bands, rings, headphones) and portable devices (mainly smartphones, which use the flash as a light source and the camera as a sensor). Thus, it becomes an affordable, convenient and widely available tool for detecting different types of arrhythmias.
Other technologies that do not rely on electrocardiographic signals are also available. These include mechanocardiography, which uses motion sensors (such as accelerometers or gyroscopes, also present in smartphones) to detect mechanical cardiac activity, and contactless photoplethysmography (also known as video plethysmography or image-based photoplethysmography), which analyzes pulsatile body signals captured by video.
SCREENING FOR ATRIAL FIBRILLATIONThe European Heart Rhythm Association recommends systematic AF screening in stroke patients, individuals older than 75 years, and those older than 65 years with comorbidities. Opportunistic screening is recommended in people over 65 years old with no comorbidities, and in younger individuals with comorbidities.5 To this end, devices based on ECG or photoplethysmography are preferred over pulse measurement; photoplethysmography has shown a high positive predictive value for diagnosing AF in large population studies.6,7 Screening is not recommended for healthy individuals younger than 65 years, although portable digital devices may be useful for patients with symptoms consistent with AF.
DIGITAL TOOLS FOR MONITORING AND FOLLOW-UP OF ARRHYTHMIA PATIENTSBeyond arrhythmia detection, these digital tools have also proven useful for patients with known AF. They can monitor heart rate, allowing remote adjustment of rate-controlling medications, and can track heart rhythms in patients attempting to maintain sinus rhythm. In this scenario, their use could assist wait-and-see strategies (outpatient treatment with deferred cardioversion) for emergency patients with recently diagnosed AF, by helping to identify those who may eventually need cardioversion due to a lack of spontaneous conversion to sinus rhythm.8 These devices could also improve the follow-up of patients undergoing ablation. Extended monitoring would provide better detection of recurrent disease than conventional strategies using in-office ECG testing or limited Holter monitoring.9
Lastly, digital tools based on mobile platforms and applications could be of help in the treatment of AF patients. They can be useful for optimizing anticoagulation, facilitating decision-making when managing symptoms, and improving control of risk factors and comorbidities through education and promotion of healthy lifestyle habits. This has a proven impact on the development of clinical events (thromboembolic events, hemorrhages, AF recurrence, symptoms, hospitalizations, and heart failure).10
Authors’ considerationsThe authors suggest using digital devices for screening AF in individuals with indications for anticoagulation therapy, such as those older than 65 years and those with comorbidities. These devices could also be useful in patients with confirmed AF for monitoring heart rate and rhythm and to provide comprehensive management to reduce clinical events. Lastly, although there is much less supporting evidence, the authors believe that digital devices, particularly ECG-based ones, could be recommended for diagnosing paroxysmal arrhythmias in patients with consistent symptoms, especially if the episodes are infrequent or of short duration.
TOOLS FOR FOLLOW UP OF HEART FAILURE PATIENTSMost of the expenditure related to heart failure (HF) management derives from outpatient visits to the emergency room and hospitalizations.11 Up to 40% of HF patients require at least 4 hospital stays during the course of their illness, and each hospitalization increases the risk of death.11 One factor contributing to repeat hospital admissions is the patients’ failure to adhere to prescribed medications, and particularly, to the recommended lifestyle changes. Adherence to these therapies tends to decrease over time after the hospital stay.12
Furthermore, as patients are often unable to recognize the early signs and symptoms of worsening, they delay seeking medical attention, which negatively affects the prognosis.
The significant recent efforts invested in telemedicine methods for HF patients have resulted in numerous benefits (table 1). These methods have proven to enhance patient adherence, predict and prevent episodes of worsening HF, and provide closer monitoring without the need to visit health care centers.
Advantages of telemedicine in heart failure
| • Improved access to specialized care, especially in rural locations or areas with a shortage of human or material resources |
| • Optimized clinical follow-up and therapy adjustments through the use of devices that can monitor parameters such as weight, blood pressure, heart rate, and blood oxygen levels, and collect information about symptoms and treatment adherence |
| • Prevention and early detection of acute decompensations |
| • Health care education and empowerment of patients through programs that promote self-care, disease knowledge, and management of risk factors |
| • Decreases in heart failure-associated health care costs by reducing unnecessary in-person visits, emergency room visits, hospitalizations, and mortality |
One part of telemedicine is telemonitoring (TM), which enables remote transmission of patients’ health data, such as symptoms, weight, heart rate, and blood pressure, stored in electronic devices. These data can then be used to seek medical advice or to adjust treatment, either directly or through a health care professional. Home-based TM can help maintain or improve the quality of care, facilitate rapid access to medical attention when needed, reduce patients’ travel expenses, and decrease the frequency of in-person appointments.13 The interruption of medical visits in many countries mandated by the COVID-19 pandemic has brought to light the potential advantages of home TM.14
Studies on home TM for predicting HF decompensation have yielded diverse results. One limitation is that adherence to the measurements tends to be incomplete, and another is that the algorithms for detecting decompensation are not entirely accurate. Systems focused on optimizing treatment and improving adherence have the advantage of only requiring staff during standard working hours, but those offering assistance demand additional resources.
The comparative effectiveness and cost-effectiveness ratio of these strategies is uncertain. However, systems focused on continuously optimizing care (the health maintenance approach) seem to be more effective than those attempting to anticipate and treat decompensation episodes. The latter approach could result in false positives, which would generate unnecessary resource consumption and demotivate health care staff due to needless alert signals.15 The development of artificial intelligence algorithms that incorporate data from various aspects of a patient's life (big data) may help to refine the alerts and make them more accurate. In addition, we should remember that TM systems can be used to effectively educate and motivate patients, but adapting and integrating them with existing health care services remains an unresolved issue in our setting.16
A 2017 Cochrane review, which identified 39 significant studies on home TM in HF patients based on assessment of symptoms, weight, heart rate, heart rhythm, and blood pressure, showed that TM was associated with a 20% reduction in all-cause mortality and a 37% reduction in HF-related hospitalizations.17 Since then, several related studies have been published, most showing no significant results and one reporting positive findings.15,18–20 However, the differences in study settings (single-center vs multicenter) and the absence of a standardized TM strategy across clinical units and patient groups may have influenced the results.
The German TIM-HF2 study20 included 1571 previously hospitalized chronic HF patients, randomly assigned to receive standard care plus daily TM or standard care alone for 12 months. The TM group experienced 21% fewer days hospitalized due to cardiovascular causes (17 vs 22 days per 100 patient-years) and 36% fewer deaths from any cause (4 vs 7 deaths per 100 patient-years) than the group without TM (both, P <.001). In the Spanish iCOR21 study, 178 HF patients were randomized to in-person follow-up or telemedicine support, which involved daily reporting of signs and symptoms through TM and structured follow-up via video or audioconferencing. The primary endpoint of the study was nonfatal HF episodes (decompensations requiring parenteral treatment) after 6 months of follow-up, which showed a 65% reduction in the TM group (0.35; 95% confidence interval [95%CI], 0.20-0.59; P <.001). In addition, HF readmissions decreased by 61% (0.39; 95%CI, 0.19-0.77; P=.007) and cardiovascular readmissions by 57% (0.43; 95%CI, 0.23-0.80; P=.008) in patients with TM. Mortality was similar in the 2 groups, and there was a significant mean net reduction in direct hospital costs of €3546 per patient. The results of this proof-of-concept report were corroborated in the multicenter HERMeS22 study, including 506 patients recently hospitalized. The preliminary results, presented at the European Heart Failure Congress in May 2023, showed a 59% reduction in the combined endpoint of cardiovascular death or worsening HF, with a number needed to treat of 4 patients.23
Specific devices for measurement and telemonitoringAlthough noninvasive, portable devices are available for monitoring heart rate and pulmonary congestion (using bioimpedance), it is not entirely clear whether they provide additional benefits compared with conventional TM.24 As for invasive TM devices, the latest implantable systems (cardiac resynchronization therapy, implantable automatic defibrillators) provide wireless remote access to information about the device itself (generator and electrode functioning), arrhythmias, and the patient's physiological data (heart rate, activity, heart sounds, bioimpedance data). These systems also incorporate algorithms for detecting congestion (eg, Optivol, Medtronic, United States and HeartLogic, Boston Scientific, United States). Several studies have shown that this type of TM is useful for detecting device malfunction and arrhythmias such as AF. However, there is no strong evidence demonstrating that these devices reduce HF hospitalizations or mortality by detecting congestion.25–27 Other implantable TM devices could also be useful, such as subcutaneous loop recorders, which can monitor heart rate and rhythm and detect activity and bioimpedance.
Devices designed for pulmonary artery implantation, such as CardioMEMS (Abbott, United States), have been developed to monitor pressure within the vessel. An increase in pulmonary artery diastolic pressure is one of the earliest signs of congestion, and detection of this increase enables timely treatment adjustments. However, the outcomes vary. Some studies show a reduction in the risk of HF-related hospitalizations28 and an improvement in quality of life.29 Others, however, such as the GUIDE-HF30 randomized clinical trial including more than 1000 stable chronic HF outpatients, found no differences in the primary outcome of all-cause mortality, hospitalization, or emergency room visits when comparing treatment based on CardioMEMS measurements to standard care. Nevertheless, the authors suggest that these results may have been influenced by the COVID-19 pandemic, as benefits were observed in the cohort enrolled before its outbreak.31
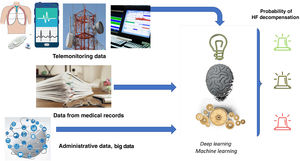
Artificial intelligenceThe most ambitious goal of eHealth in HF may be the anticipation of decompensations. Artificial intelligence with big data-based prediction algorithms can be a valuable complement to provide additional benefits to TM. A recent review32 described the performance of artificial intelligence-based algorithms for predicting hospital admissions in HF patients. Among the studies analyzed, 16 predicted hospital readmissions within 30 days and over periods ranging from 6 months to 3 years, with areas under the curve of 0.61 to 0.79. The most accurate predictions were observed in studies including TM parameters. For example, a prospective study evaluating a disposable sensor patch worn at home post-hospitalization achieved an area under the curve of 0.89 for predicting admission.33 The study included a 7-day patch that continuously recorded ECG waveform, 3-axis accelerometry, skin impedance and temperature, and provided information on activity and posture. The data obtained were integrated with the patient's administrative data and medical history, and analyzed using machine learning and deep learning models (figure 3).
Central illustration. Diagram showing the process of integrating different data from various sources and analyzing them using machine learning and deep learning models. Prepared with data from Stehlik et al.33 HF, heart failure.
Other artificial intelligence-related telemedicine options in HF involve speech analysis for detecting pulmonary fluid overload. An application for mobile devices has been developed that successfully identifies clinical conditions affecting the mechanics of vocal cord vibrations through the use of voice sound analysis. By recording just 6 phrases daily, the HearO application (Cordio, Israel) detected more than 90% of voice changes indicating increased congestion.34 HearO is a speech processing and analysis application able to distinguish speech characteristics at hospital admission (wet) from those at discharge (dry). In a recent preliminary analysis of data from 253 HF outpatients, the application detected 80% of decompensation episodes with a mean lead time of 22.5 days compared to the conventional strategy, and with very few false positives (2.8 false alerts per year).
Authors’ considerationsBased on the available data mentioned above, the clinical practice guidelines of the European Society of Cardiology35 suggest that TM can be used both noninvasively and invasively (pulmonary artery pressure) with a low recommendation (IIb) and level of evidence B (table 2). We suggest that the studies conducted, the lack of standardized methods, poor adaptation to local contexts, difficulties in integrating the techniques into existing computer systems (data security and confidentiality), insufficient funding, and a lack of incentives for real-life use do not adequately reflect the potential value of telemedicine and TM. In the setting of HF, we believe that telemedicine, including TM, should be used as follows: a) to assist the prompt detection of decompensations, b) to motivate patients and improve their treatment adherence, and c) to educate patients about their condition. Furthermore, even if TM does not outperform in-person patient visits in terms of health benefits and is only similarly effective, the overall balance of its inclusion would be positive (convenience for patients, time and resource savings). In the authors’ opinion, the recent results from the multicenter HERMeS22 study, which showed a very small number of patients needed-to-treat to prevent an event, should carry substantial weight in future guidelines to strengthen this recommendation.
Recommendations for telemonitoring in heart failure
| Recommendations | Class | Level |
|---|---|---|
| Noninvasive TM may be considered for HF patients to reduce the risk of recurrent hospitalizations due to HF and cardiovascular conditions, and the risk of cardiovascular death | IIb | B |
| Monitoring of pulmonary arterial pressure based on wireless hemodynamic systems may be considered for HF patients to improve clinical outcomes | IIb | B |
Recommendations for telemonitoring in heart failure according to the European guidelines on heart failure, as per McDonagh et al.35 HF, heart failure; TM, telemonitoring.
Telemedicine can help with addressing risk factors, improving treatment adherence, and managing symptoms. The process of modifying risk factors includes monitoring and improving blood pressure and lipid levels, promoting exercise and dietary changes, and providing counseling to support smoking cessation.36 Simple tools such as phone calls, short text messages, and online portals can be used to track vital signs and laboratory results, adjust dosage, and encourage physical activity, proper diet, and treatment compliance. Results from small randomized controlled trials and meta-analyses have shown a significant improvement in risk factors with telemedicine, although the long-term effectiveness of the interventions remains uncertain.36–38 Comprehensive approaches to cardiovascular risk factors have been extensively assessed in cardiac rehabilitation programs, and the guidelines recommend telemedicine as a valid follow-up option to enhance long-term adherence (recommendation level IIb, evidence level B).39 However, the role of telemedicine in cardiac rehabilitation is beyond the scope of this article.
Digital tools for hypertensionDigital devices for blood pressure measurementResearch into blood pressure monitoring focuses on two main issues: the first is to make existing cuff-based measurement systems easier to use and better suited to use in daily life, while the second seeks to develop a technique that uses readily accessible biomedical signals to measures blood pressure with reasonable accuracy. Regarding the first issue, the main goal for newer devices is to make them more compact, efficient, and robust, as their precision level is already sufficient. The main concerns regarding the second issue are device accuracy and resolving complexities such as their applicability in different age groups and other factors.40
Similar to developments in heart rate monitoring, there is an effort to achieve continuous noninvasive blood pressure monitoring with devices that do not rely on plethysmography and pulse waves.41
Nonetheless, these cuffless devices must show better performance before they can be recommended for clinical use. Currently, there are substantial concerns regarding their reproducibility, and they still require validation.42 For these reasons, scientific societies have not recommended them43 up to now, and methods for validation have recently emerged44 to address these concerns.
Devices for improving blood pressure managementCertain devices can assist in blood pressure control and remote monitoring based on mobile applications that combine telemedicine and health education. There are many recent examples. A systematic review found that home TM for blood pressure monitoring with automatic data transmission can be more effective than conventional hypertension therapy, with studies using smartphone applications showing particularly beneficial effects.45 Another recent example of the role of these applications in blood pressure management is the HERB DH1 study.46
These TM strategies have been successfully incorporated into prevention programs together with management of other risk factors (such as lipid levels) and have led to a reduction in visits to health care centers.47
Digital tools for dyslipidemiaSeveral studies have shown that control of low-density lipoprotein cholesterol in secondary prevention is often insufficient.48 Telemedicine-based solutions may offer an opportunity to improve this situation. One approach that has provided beneficial results is the concept of virtual lipid visits49 based on rigorous virtual follow-up (electronic medical records and phone calls) after hospital discharge, with consecutive analyses and adjustment of lipid-lowering therapy. This strategy has shown effective control of low-density lipoprotein cholesterol following an acute coronary syndrome.
These TM strategies for managing lipid levels have been included in prevention programs together with the treatment of other risk factors such as hypertension, with proven success.47
Digital tools for diabetesDigital devices for glucose measurementWell-established invasive or direct devices for continuous glucose measurement are currently in use for type 1 and type 2 diabetes.50
There are now ongoing efforts to develop noninvasive methods for glucose assessment51 that will likely become available to consumers in the same way as devices for arrhythmia detection.52 Hence, it is important to be well-informed about these technologies to assess their future use and determine the appropriate indications.
Digital tools for managing diabetesDigital tools in the form of applications have been proven to enhance diabetes control in several ways,53 particularly by influencing lifestyle.54 The benefits seem to be more evident in younger patients and in systems that provide feedback with health care personnel.
Digital tools to address smokingIt is essential to address smoking in cardiovascular conditions,55 and telemedicine has proven to be of considerable value in the setting of respiratory diseases.56 There is evidence that telemedicine-based strategies using messaging can help individuals stop smoking.57
Digital tools to promote adherenceTreatment adherence is vital in the management of cardiovascular diseases.58 Strategies such as polypill drug combinations59 have been used for this purpose, but there may also be a role for telemedicine-based approaches.
Several reports have described strategies to improve adherence using methods that range from messaging, which has proven unsuccessful,60 to approaches that integrate various tools, which have shown some success.61
Authors’ considerationsAt this time, we do not recommend cuffless devices for blood pressure monitoring. Instead, we suggest TM with automatic transmission of blood pressure values, particularly systems based on applications and integration with clinical records. To improve lipid control, we suggest implementing strategies and algorithms in the clinical records that can trigger alerts, such as phone calls or messaging reminders. We do not recommend noninvasive devices for glucose monitoring; when accurate monitoring is required, invasive devices should be used. To enhance diabetes control, smoking cessation, and treatment adherence, we suggest using application-based systems, validated by clinical studies. We do not recommend strategies that rely solely on messaging to improve adherence.
FUTURE CHALLENGESFuture challenges that should be addressed in telemedicine for cardiovascular diseases include:
- •
Ensuring that patients have universal access to the necessary technology and knowledge of its use.
- •
Establishing privacy and data security measures to protect this information.
- •
Promoting a multidisciplinary approach (nursing, primary care, etc.) with seamless integration and interaction with the medical history.
- •
Generating additional evidence that assesses the quality of care, clinical outcomes, and patient satisfaction.
- •
Providing adequate training to both patients and professionals in telemedicine use, and imparting knowledge of its benefits and limitations.
- •
Integrating telemedicine with artificial intelligence and big data, which will enable more personalized medical care, timely detection, support for clinical decision-making, and remote patient monitoring.
None.
AUTHORS’ CONTRIBUTIONSAll the authors have substantially contributed to the conception and design, acquisition of data, and writing of the article, as well as critical revision of its intellectual content. They have approved the final version and agree to take responsibility for all aspects of the article, and to investigate and resolve any issues related to the accuracy and truthfulness of any part of it.
CONFLICTS OF INTERESTNone.