Pharmacoinvasive strategy represents an attractive alternative to primary angioplasty. Using cardiovascular magnetic resonance imaging we compared the left ventricular outcome of the pharmacoinvasive strategy and primary angioplasty for the reperfusion of ST-segment elevation myocardial infarction.
MethodsCardiovascular magnetic resonance was performed 1 week and 6 months after infarction in two consecutive cohorts of patients included in a prospective university hospital ST-segment elevation myocardial infarction registry. During the period 2004–2006, 151 patients were treated with pharmacoinvasive strategy (thrombolysis followed by routine non-immediate angioplasty). During the period 2007–2008, 93 patients were treated with primary angioplasty. A propensity score matched population was also evaluated.
ResultsAt 1-week cardiovascular magnetic resonance, pharmacoinvasive strategy and primary angioplasty patients showed a similar extent of area at risk (29±15 vs. 29±17%, P=.9). Non-significant differences were detected by cardiovascular magnetic resonance at 1 week and at 6 months in infarct size, salvaged myocardium, microvascular obstruction, ejection fraction, end-diastolic volume index and end-systolic volume index (P>.2 in all cases). The same trend was observed in 1-to-1 propensity score matched patients. The rate of major adverse cardiac events (death and/or re-infarction) at 1 year was 6% in pharmacoinvasive strategy and 7% in primary angioplasty patients (P=.7).
ConclusionsA pharmacoinvasive strategy including thrombolysis and routine non-immediate angioplasty represents a widely available and logistically attractive approach that yields identical short-term and long-term cardiovascular magnetic resonance-derived left ventricular outcome compared to primary angioplasty.
Keywords
Primary angioplasty (PA) has become the preferred therapy in the reperfusion of ST-segment elevation myocardial infarction (STEMI). However, PA is not universally available and thrombolysis is still the predominant reperfusion treatment in many western countries.1,2,3
A pharmacoinvasive strategy (PI), namely thrombolysis followed by rescue angioplasty if needed or routine (but not immediate) angioplasty after thrombolysis, appears to be a widely accessible and easily implemented approach. This policy is especially useful in areas far from tertiary hospitals; theoretically, it combines the beneficial effects of a timely reperfusion with thrombolytic agents and the resolution of residual coronary stenosis by means of early, but not immediate, angioplasty.2,3,4 Recent registries have shown that PI and PA result in a comparable patient outcome5,6,7,8 and both strategies have been accepted in current guidelines for the management of STEMI patients.9,10
It could be speculated that the equivalence of PI and PA strategies regarding patient outcome could be the result of a comparable effect in terms of systolic function, left ventricular dilation, infarct size, salvaged myocardium and microvascular obstruction. Cardiovascular magnetic resonance (CMR) permits, in a single session, a comprehensive state-of-the-art evaluation of all these parameters in STEMI patients.11,12 To date, a head-to-head comparison of the CMR-derived left ventricular outcome of PI and PA has not been carried out.
The objective of the present study was to use CMR to compare the short-term and long-term left ventricular repercussion of PI and PA strategies.
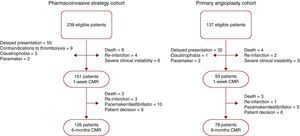
MethodsFrom January 2004 to December 2008 we prospectively included 375 patients admitted to our institution during regular working hours with a first STEMI treated with reperfusion therapies and evaluated with CMR at pre-discharge. Exclusion criteria were: contraindications to CMR (n=8), death (n=12), re-infarction (n=6), severe clinical instability (n=9), delayed presentation (>12hours after chest pain onset, n=87) and contraindications to thrombolysis (n=9). Therefore, the final study group comprised 244 patients with a first STEMI treated with reperfusion therapies and studied with CMR at pre-discharge (Figure 1).
Figure 1. Flow chart of patients included in the pharmacoinvasive and in the primary angioplasty cohorts. CMR, cardiovascular magnetic resonance.
According to our institution's protocol for management of STEMI patients during regular working hours, a PI strategy was applied from 2004-2006; PA has been the routine approach since 2007. To assess the effects of reperfusion therapies, CMR was routinely performed at pre-discharge and at 6 months in all patients assigned to the treatment protocol established for each period.
Medical treatment was left at the discretion of the attending cardiologist. Baseline characteristics and clinical data were recorded in all cases. The Thrombolysis in Myocardial Infarction (TIMI) risk score for STEMI was calculated in all patients as a surrogate of baseline clinical risk.13 The percentage of ST-segment resolution 90min after reperfusion therapy was determined using previously validated methodology.14 Troponin I (Dimension; Dade Behring, Newark, New Jersey, USA) was serially measured and peak troponin I was assessed. Time since chest pain onset and since diagnosis to revascularization (thrombolytic therapy infusion in PI, balloon inflation in PA) were recorded.
The local ethics committee approved the research protocol. Written informed consent was obtained from all subjects.
Study cohorts Pharmacoinvasive strategy cohortFrom January 2004 to December 2006 the reperfusion strategy consisted of the administration of full-dose tenecteplase plus enoxaparin within the first 12hours after chest pain onset. In the case of persistent chest pain or ST-segment resolution <70% at 90minutes after thrombolytic therapy, rescue angioplasty was carried out. In the case of successful thrombolytic therapy, namely absence of chest pain and ST-segment resolution >70% at 90minutes after thrombolytic therapy, routine angioplasty was performed at least 3hours afterwards. Of the 238 patients admitted for STEMI during the PI period, the final PI cohort included 151 patients. At 6 months, 126 patients were reevaluated with CMR. The flow chart of patients is shown in Figure 1.
Primary angioplasty cohortFrom January 2007 to December 2008 PA was the routine reperfusion strategy in our institution. Of the 137 patients admitted for STEMI during the PA period, the final PA cohort included 93 cases. At 6 months, 78 patients were reevaluated with CMR. The flow chart of patients is shown in Figure 1.
Cardiac CatheterizationMedical treatment and invasive management in the catheterization lab were left to the discretion of the attending interventional cardiologist. Procedures were performed in a high-volume cardiac catheterization facility with 24/7 percutaneous revascularization capability by 3 experienced interventionalists (each having performed >1000 percutaneous revascularization procedures, >300 of them in STEMI patients). TIMI flow grade and myocardial blush grade were determined offline by an experienced observer using standard software (Integris HM3000, Philips, Best, The Netherlands). TIMI flow grade 3 and myocardial blush grade 2-3 were regarded as normal.
Cardiovascular Magnetic ResonanceIn accordance with our laboratory protocol and current recommendations,11,12,15 CMR (1.5-T scanner, Sonata Magnetom, Siemens, Erlangen, Germany) was performed at 7±1 days and 181±11 days after STEMI. Steady-state free precession sequences were used for cine imaging, a dark-blood T2-weighted short-tau inversion-recovery turbo-spin echo sequence was applied to determine the area at risk (with edema) and a segmented inversion recovery steady-state free precession sequence was used for late enhancement imaging. Further details regarding our CMR protocol can be consulted elsewhere.11,12 All images were acquired by a phased-array body surface coil during breath-holds and were ECG-triggered.
CMR studies were analyzed offline by an experienced observer blinded to all patient data, using customized software (QMASS MR 6.1.5, Medis, Leiden, The Netherlands).
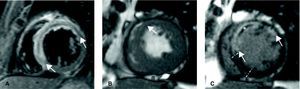
End-diastolic volume index (ml/m2), end-systolic volume index (ml/m2), ejection fraction (%) and left ventricular mass (g/m2) were quantified by manual definition of endocardial and epicardial borders of all short-axis slices in cine images (Figure 2). For dichotomous analyses, end-diastolic volume index and end-systolic volume index were considered to be dilated and ejection fraction was considered to be depressed on the basis of accepted reference values according to sex, age and body surface area.16
Figure 2. Cardiovascular magnetic resonance images at 1 week in a patient with a large anterior myocardial infarction. A: T2-weighted sequence demonstrated a large area at risk (with edema, between arrows). B: cine images demonstrated hypokinesia in the anterior area (arrow). C: late enhancement imaging allowed for the definition of infarct size (hyper-enhanced myocardium, between arrows) and microvascular obstruction (dark area in the middle of infarcted tissue, asterisk). By comparing area at risk and infarct size, salvaged myocardium was considered as the area at risk not showing late enhancement (between bars).
Area at risk (with edema) was quantitatively defined in T2-weighted images as the percentage of left ventricular mass with signal intensity 2 standard deviations above the mean signal obtained in the remote non-infarcted myocardium (Figure 2, Figure 3).
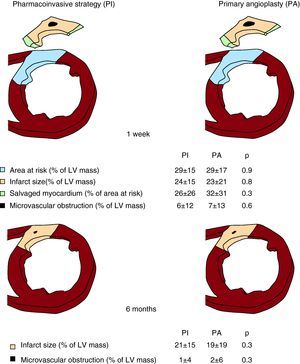
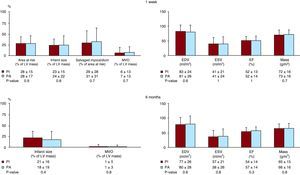
Figure 3. T2-weighted and late enhancement imaging results in the whole study group at 1 week and 6 months. Area at risk, infarct size, salvaged myocardium and microvascular obstruction in patients treated with the pharmacoinvasive strategy (left) and with primary angioplasty (right). Upper panels: first week. Lower panels: six months. Area at risk, infarct size and microvascular obstruction are expressed as the percentage of left ventricular mass. Salvaged myocardium is expressed as the percentage of area at risk. LV, left ventricle; PA, primary angioplasty; PI, pharmacoinvasive strategy.
In delayed enhancement imaging, infarct size was quantitatively determined as the percentage of left ventricular mass with signal intensity 2 standard deviations above the mean signal obtained in the remote non-infarcted myocardium (Figure 2, Figure 3). Area at risk and infarct size were visually reviewed by the operator and manually corrected if needed.
Salvaged myocardium was regarded as the percentage of area at risk not showing delayed enhancement (Figure 2, Figure 3).
Microvascular obstruction was defined, by manual planimetry, as the percentage of left ventricular mass displaying a lack of contrast uptake in the core of an area showing delayed enhancement (Figure 2, Figure 3).
In our laboratory, intraobserver variability for left ventricular volume indexes, ejection fraction, area at risk, infarct size, salvaged myocardium and microvascular obstruction is <5%.17
End-PointsThe primary end-point was to compare area at risk, infarct size, salvaged myocardium, microvascular obstruction, end-diastolic volume index, end-systolic volume index and ejection fraction in the PI and PA cohorts at the 1-week CMR.
The secondary end-point was to compare infarct size, microvascular obstruction, end-diastolic volume index, end-systolic volume index and ejection fraction in the PI and PA cohorts at the 6-month CMR.
Clinical Follow-upThis study did not include clinical end-points. We computed the rate of major adverse cardiac events (MACEs), ie, post-discharge death and/or re-infarction, whichever occurred first; major hemorrhages; and re-admission for heart failure at 1-year follow-up. Three cardiologists reviewed all events and consensus was required to designate a clinical event.
Statistical AnalysisThe Kolmogorov-Smirnov test was applied to test for normal distribution. Continuous variables were expressed as mean±1 SD or median (quartile 1 - quartile 3) when appropriate. For continuous normally distributed variables, comparisons were calculated using the unpaired t test and the one-way ANOVA test. For continuous non-normally distributed variables, comparisons were calculated using the Mann-Whitney U test. Group percentages were compared using the Chi-square test or Fisher's exact test when appropriate. Survival distributions for time-to-MACE of PI and PA patients were estimated using the Kaplan-Meier method and the log-rank test. Statistical significance was considered for a two-tailed p<0.05. The SPSS statistical package (version 13.0, SPSS Inc., Chicago, Illinois, USA) was used.
Due to the observational design of the study, and to minimize any potential bias, we repeated all comparisons in a 1-to-1 propensity score matched population that included 80 PI and 80 PA patients. To estimate the propensity score, we performed a logistic regression analysis with PA as the dependent variable. Matched patients showing the same value in the logistic regression model were included in this substudy. Psmatch2 module (STATA 11, StataCorp, College Station, Texas, USA) was used for this purpose.
ResultsThe baseline characteristics of the 151 PI and the 93 PA patients are shown in Table 1. The PI and PA cohorts were comparable, with the exception of the expected differences: longer time to revascularization, higher rates of normal TIMI and myocardial blush grades at the first angiography (before stent), and lesser use of IIbIIIa inhibitors in PI patients (Table 1). Median (range) time to revascularization was 180 (35-610) minutes in PI and 212 (50-710) minutes in PA patients (p<0.001).
Table 1. Characteristics of Patients Treated With the Pharmacoinvasive Strategy (PI) and With Primary Angioplasty (PA) *
| PI | PA | p | |
| Number of patients | 151 | 93 | |
| Baseline characteristics | |||
| Age (years) | 58±11 | 60±13 | 0.2 |
| Male sex (%) | 128 (85) | 75 (81) | 0.5 |
| Diabetes (%) | 22 (15) | 14 (15) | 1 |
| Hypertension (%) | 66 (44) | 43 (46) | 0.8 |
| Hypercholesterolemia (%) | 56 (37) | 40 (43) | 0.4 |
| Smoker (%) | 93 (62) | 50 (54) | 0.2 |
| Anterior infarction (%) | 86 (57) | 47 (51) | 0.4 |
| Heart rate (bpm) | 81±22 | 80±17 | 0.6 |
| Systolic pressure (mm Hg) | 126±27 | 125±26 | 0.8 |
| Killip class I (%) | 134 (89) | 84 (90) | 1 |
| Killip class II (%) | 13 (9) | 7 (8) | 0.9 |
| Killip class III (%) | 4 (2) | 2 (2) | 1 |
| Killip class IV (%) | 0 | 0 | 1 |
| Time to reperfusion (min) | 180 (120-270) | 212 (140-394) | <0.001 |
| Diagnosis to reperfusion (min) | 60 (37-93) | 76 (42-124) | <0.001 |
| TIMI risk score | 3±2 | 3±2 | 1 |
| Peak troponin I (ng/ml) | 82 (44-100) | 77 (38-99) | 0.7 |
| ST-segment resolution (%) | 78±21 | 82±23 | 0.1 |
| Cardiac catheterization | |||
| Proximal left anterior descending (%) | 40 (27) | 27 (29) | 0.7 |
| Multivessel disease (%) | 33 (20) | 19 (20) | 1 |
| Treatment with stent (%) | 132 (87) | 89 (96) | 1 |
| TIMI flow grade 0 pre-stent (%) | 19 (13) | 50 (54) | <0.001 |
| TIMI flow grade 1 pre-stent (%) | 20 (13) | 21 (22) | 0.09 |
| TIMI flow grade 2 pre-stent (%) | 18 (12) | 11 (12) | 1 |
| TIMI flow grade 3 pre-stent (%) | 94 (62) | 11 (12) | <0.001 |
| TIMI flow grade 3 post-stent (%) | 136 (90) | 85 (91) | 0.8 |
| Blush grade 2-3 pre-stent (%) | 74 (49) | 8 (9) | <0.001 |
| Blush grade 2-3 post-stent (%) | 118 (78) | 67 (72) | 0.3 |
| In-hospital medical treatment | |||
| Aspirin | 151 (100) | 93 (100) | 1 |
| Clopidogrel | 151 (100) | 93 (100) | 1 |
| IIbIIIa inhibitors (%) | 35 (24) | 86 (92) | <0.001 |
| Beta-blockers (%) | 85 (57) | 48 (52) | 0.5 |
| ACE inhibitors (%) | 79 (53) | 38 (40) | 0.09 |
| Statins (%) | 113 (76) | 65 (70) | 0.4 |
| Diuretics (%) | 14 (9) | 14 (15) | 0.2 |
Abbreviations: ACE, angiotensin converting enzyme; bpm, beats per minute; TIMI, Thrombolysis in Myocardial Infarction.
* Continuous variables with a normal distribution are expressed as mean±standard deviation and those without a normal distribution are expressed as median (quartile 1- quartile 3).
All PI cases underwent coronary angiography within the first 48hours. Of the 151 patients, 35 (23%) were treated with rescue angioplasty (202±129min after thrombolytic therapy) and 97 (64%) with routine post-thrombolysis angioplasty (median 18hours, range 3 to 47hours after thrombolytic therapy). Overall, 132 PI patients (87%) received stent therapy within the first 2 days. Nineteen patients (13%) were not treated with stents because of failed angioplasty (n=4), absence of significant residual stenosis in the culprit artery after thrombolysis (n=12), or surgical revascularization (n=3).
In the PA strategy, stents were implanted in 89 patients (96%); 3 patients were not treated with stents because of failed PA in 2 and surgical revascularization in 1.
One-Week Cardiovascular Magnetic ResonanceCMR characteristics of PI and PA patients are shown in Figure 3, Figure 4. At the 1-week CMR, area at risk, infarct size, salvaged myocardium, microvascular obstruction, end-diastolic volume index, end-systolic volume index and ejection fraction were similar in PI and PA patients. Microvascular obstruction was detected in 69 PI patients (46%) and in 43 (47%) PA patients (p=0.9).
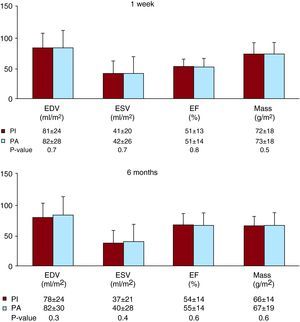
Figure 4. Cardiovascular magnetic resonance results in the whole study group at 1 week and 6 months. Left ventricular volumes, ejection fraction (EF) and mass in patients treated with the pharmacoinvasive strategy (PI) and with primary angioplasty (PA). Upper panel: first week. Lower panel: six months. EDV, end-diastolic volume; ESV: end-systolic volume.
According to previously validated cut-off values, PI (n=151) and PA (n=93) patients displayed similar rates of dilated end-diastolic volume index (24% vs. 24%, p=1), dilated end-systolic volume index (44% vs. 44%, p=1) and depressed ejection fraction (62% vs. 59%, p=0.8), respectively, at 1 week.
Six-Month Cardiovascular Magnetic ResonanceCMR characteristics of the 126 PI and 78 PA patients re-studied at 6 months are displayed in Figure 3, Figure 4. At 6 months no patient displayed myocardial edema (area at risk). Overall, no significant differences were detected between the two cohorts regarding infarct size, microvascular obstruction, end-diastolic volume index, end-systolic volume index and ejection fraction at the 6-month CMR.
In parallel to 1-week CMR results, PI and PA patients displayed similar rates of dilated end-diastolic volume index (19% vs. 21%, p=0.7), dilated end-systolic volume index (37% vs. 38%, p=0.9) and depressed ejection fraction (51% vs. 46%, p=0.4), respectively, at 6 months.
Rescue AngioplastyPatients treated with rescue angioplasty (n=35) were separately evaluated (Table 2). Rescue angioplasty was associated with CMR indexes that were more altered than those observed in patients with successful thrombolysis (n=116) and primary angioplasty (n=93).
Table 2. Cardiovascular Magnetic Resonance (CMR) Characteristics of Patients Treated With Successful Thrombolysis, With Rescue Angioplasty Because of Unsuccessful Thrombolysis, and With Primary Angioplasty *
| Successful thrombolysis | Primary angioplasty | Rescue angioplasty | p for trend | |
| Number of patients | 116 | 93 | 35 | |
| 1-week CMR | ||||
| Ejection fraction (%) | 53±13 | 51±14 | 45±12 | 0.01 |
| End-diastolic volume (ml/m2) | 80±23 | 82±28 | 85±25 | 0.5 |
| End-systolic volume (ml/m2) | 39±19 | 42±26 | 48±22 | 0.09 |
| LV mass (g/m2) | 70±16 | 73±20 | 78±24 | 0.05 |
| Area at risk (% of LV mass) | 27±15 | 29±17 | 36±16 | 0.01 |
| Infarct size (% of LV mass) | 22±15 | 23±21 | 31±15 | 0.03 |
| Salvaged myocardium (% of area at risk) | 28±27 | 32±31 | 19±22 | 0.08 |
| Microvascular obstruction (% of LV mass) | 5±12 | 7±13 | 11±12 | 0.06 |
| 6-month CMR | ||||
| Ejection fraction (%) | 55±14 | 55±14 | 50±13 | 0.2 |
| End-diastolic volume (ml/m2) | 75±23 | 82±30 | 87±24 | 0.07 |
| End-systolic volume (ml/m2) | 35±20 | 40±28 | 46±22 | 0.08 |
| LV mass (g/m2) | 65±14 | 67±19 | 68±14 | 0.2 |
Abbreviations: CMR, cardiovascular magnetic resonance; LV, left ventricle.
* Data expressed as mean±standard deviation.
At 1-year follow-up, no significant differences were detected between PI and PA patients in rate of cardiac death (5/151, 3% vs. 5/93, 5%, p=0.3), re-infarction (5/151, 3% vs. 4/93, 4%, p=0.7) and first MACE (9/151, 6% vs. 6/93, 7%, p=0.8) (Figure 5). The rates of major hemorrhages (2/151, 1% vs. 1/93, 1%, p=0.8) and re-admission for heart failure (13/151, 9% vs. 6/93, 7%, p=0.6) were similar between PI and PA patients.
Figure 5. Reperfusion strategies and major adverse cardiac events. Kaplan-Meier survival distributions without major adverse cardiac events (death or nonfatal myocardial infarction) in patients treated with the pharmacoinvasive strategy and with primary angioplasty.
Propensity Score-Matching SubanalysisNo significant differences existed between 1-to-1 matched PA (n=80) and PI (n=80) patients in terms of baseline characteristics, post-procedure angiographic data and in-hospital management, with the exception of more frequent use of IIbIIIa inhibitors in PA patients (Table 3).
Table 3. Characteristics of Patients Included in The Propensity Score Match Subanalysis and Treated With The Pharmacoinvasive Strategy (PI) and With Primary Angioplasty (PA) *
| PI | PA | p | |
| Number of patients | 80 | 80 | |
| Baseline characteristics | |||
| Age (years) | 58±11 | 60±13 | 0.3 |
| Male sex (%) | 69 (86) | 64 (80) | 0.4 |
| Diabetes (%) | 10 (13) | 13 (16) | 0.7 |
| Hypertension (%) | 28 (35) | 40 (50) | 0.08 |
| Hypercholesterolemia (%) | 31 (39) | 34 (43) | 0.7 |
| Smoker (%) | 49 (61) | 45 (56) | 0.6 |
| Anterior infarction (%) | 45 (56) | 41 (51) | 0.6 |
| Heart rate (bpm) | 81±21 | 78±16 | 0.5 |
| Systolic pressure (mm Hg) | 129±29 | 126±27 | 0.3 |
| Killip class I (%) | 72 (90) | 70 (87) | 0.9 |
| Killip class II (%) | 6 (7) | 7 (9) | 0.9 |
| Killip class III (%) | 2 (2) | 3 (4) | 0.7 |
| Killip class IV (%) | 0 | 0 | 1 |
| Time to reperfusion (min) | 180 [136-286] | 192 [132-207] | 0.07 |
| Diagnosis to reperfusion (min) | 60 [35-98] | 76 [42-129] | 0.01 |
| TIMI risk score | 3±2 | 3±2 | 1 |
| Peak troponin I (ng/ml) | 78 [43-100] | 80 [39-100] | 0.7 |
| ST-segment resolution (%) | 78 (23) | 81 (23) | 0.3 |
| Cardiac catheterization | |||
| Proximal left anterior descending (%) | 21 (26) | 23 (29) | 0.9 |
| Multivessel disease (%) | 16 (20) | 15 (19) | 1 |
| Treatment with stent (%) | 76 (96) | 77 (96) | 1 |
| TIMI flow grade 0 pre-stent (%) | 11 (14) | 40 (50) | <0.001 |
| TIMI flow grade 1 pre-stent (%) | 9 (11) | 19 (24) | 0.08 |
| TIMI flow grade 2 pre-stent (%) | 12 (15) | 10 (12) | 0.8 |
| TIMI flow grade 3 pre-stent (%) | 48 (60) | 11 (14) | <0.001 |
| TIMI flow grade 3 post-stent (%) | 67 (84) | 72 (90) | 0.4 |
| Blush grade 2-3 pre-stent (%) | 35 (43) | 8 (10) | <0.001 |
| Blush grade 2-3 post-stent (%) | 62 (78) | 60 (75) | 0.9 |
| Medical treatment | |||
| Aspirin | 80 (100) | 80 (100) | 1 |
| Clopidogrel | 80 (100) | 80 (100) | 1 |
| IIbIIIa inhibitors (%) | 17 (21) | 72 (90) | <0.001 |
| Beta-blockers (%) | 45 (56) | 40 (50) | 0.5 |
| ACE inhibitors (%) | 44 (55) | 33 (41) | 0.1 |
| Statins (%) | 64 (80) | 54 (67) | 0.1 |
| Diuretics (%) | 10 (13) | 11 (14) | 1 |
Abbreviations: ACE, angiotensin converting enzyme; bpm: beats per minute; TIMI, Thrombolysis in Myocardial Infarction.
* The figures express n (%), mean or median [interquartile range].
Area at risk, infarct size, salvaged myocardium, microvascular obstruction, end-diastolic volume index, end-systolic volume index and ejection fraction at 1 week and at 6 months were almost identical in PI and PA patients (Figure 6).
Figure 6. Cardiovascular magnetic resonance results in the propensity score matched groups. Area at risk, infarct size, salvaged myocardium, microvascular obstruction, left ventricular volumes, ejection fraction (EF) and mass in patients treated with the pharmacoinvasive strategy (PI) and with primary angioplasty (PA). Upper panels: first week. Lower panels: six months. EDV, end-diastolic volume; ESV, end-systolic volume; LV, left ventricle; MVO, microvascular obstruction.
The rates of 1-year cardiac death (6% vs. 6%, p=1), re-infarction (4% vs. 4%, p=1), first MACEs (9% vs. 8%, p=0.5), major hemorrhages (1% vs. 1%, p=1) and re-admission for heart failure (7% vs. 5%, p=0.7) were similar in PI and PA patients.
DiscussionThe main finding of the present study is that a PI strategy based on thrombolytic treatment and routine non-immediate angioplasty affords short- and long-term results in terms of CMR-derived left ventricular repercussion that are equivalent to PA outcomes.
Reperfusion StrategiesOn the basis of multiple randomized clinical trials demonstrating superiority of rapid PA over thrombolysis, PA has become the preferred approach to management of STEMI.1,9,10 However, many patients do not meet criteria for appropriate PA use. Factors related to delayed reperfusion (whether due to patient transfer, lack of permanently available skilled interventional groups, or budget limitations) explain why thrombolysis still remains the common reperfusion therapy in many areas.2,3,4 Even in institutions like ours, with a primary angioplasty program, this therapy carries with it an inherent delay compared to thrombolytic treatment.
Thrombolysis relates to a higher risk of re-infarction and a lower probability of TIMI 3 flow, which represents its Achilles’ heel in comparison with PA.2,3 Several combinations have been tested2,3,4,5,6,7,8,9,10 in efforts to combine the speed of thrombolysis in achieving coronary reperfusion with the effectiveness of angioplasty in completely opening residual stenosis and sealing unstable plaques.
Facilitated angioplasty represents a pathophysiologically attractive strategy consisting of thrombolysis followed by immediate angioplasty.2,3,18 However, the largest study to date comparing facilitated angioplasty with PA (ASSENT-4 PCI)18 was terminated early due to increased in-hospital mortality in the facilitated arm (6% vs. 3%). These disappointing results might be due to the fact that very early percutaneous revascularization of highly unstable plaques with a large thrombotic burden could worsen the coronary flow successfully recovered by thrombolysis.2,3,4 As a consequence, facilitated angioplasty cannot be recommended in the management of STEMI patients.9,10
Pharmacoinvasive StrategyRecent studies using a PI studies of early thrombolytic infusion followed by routine non-immediate angioplasty have obtained excellent results, comparable to PA, in terms of patient outcome. In contrast to the first studies comparing PA and thrombolysis (where subsequent angioplasty was unfrequently performed), in the new series of PI strategy more than 80% of patients underwent pre-discharge angioplasty.5,6,7,8 On the basis of the poor results of facilitated angioplasty and the encouraging outcome of the PI strategy, it seems that from 3 to 24h following thrombolysis represents the ideal timing for routine post-thrombolysis angioplasty.9,10 Theoretically, PI combines the beneficial effects of timely reperfusion with thrombolytic agents and the resolution of residual coronary stenosis by means of angioplasty of a plaque with less instability and thrombotic burden than in the case of facilitated angioplasty.2,3,4
We studied two consecutive cohorts included in a prospective STEMI registry. The first cohort comprised 151 STEMI patients managed with a PI strategy that included in-hospital thrombolysis followed by angiography within the first 2 days in all cases and percutaneous revascularization in 87% (rescue angioplasty in 23%). The PA cohort included 93 patents and 96% of them were treated with stent. Although clinical outcome was not the objective of the present study, we observed that the MACEs rate at 1 year was almost identical in both strategies (PI 6%, PA 7%). Thus our data supports the concept that, in terms of clinical end-points, a PI strategy including a broad use of pre-discharge revascularization can achieve similar results to PA.
Unsuccessful thrombolysis represents the main limitation of the PI strategy. Even though rescue angioplasty is a common practice in these cases, its beneficial effects in terms of left ventricular repercussion remain unclear. Actually, in our series, patients treated with rescue angioplasty displayed the most severe changes in CMR indexes both at 1 week and at 6 months.
Left Ventricular Repercussion in Pharmacoinvasive Strategy and Primary Angioplasty PatientsThe ultimate goal of all reperfusion strategies is to minimize myocardial damage and eventually to preserve systolic function and limit left ventricular remodeling.1,2,3 The deleterious effects of depressed systolic function and dilated left ventricular volumes on patient outcome have been solidly demonstrated.9,10,19
Data comparing the left ventricular repercussion of PI strategy vs. PA is scarce. In the GRACIA-2 study, Fernandez-Aviles et al reported similar ejection fraction, left ventricular volumes and regional systolic dysfunction at 6-week angiography in patients managed with the PI strategy and with PA.7
In a single session, CMR permits comprehensive evaluation of a wide variety of indexes and is now considered the gold standard technique for the noninvasive assessment of STEMI patients.11,15,19 The present study is the first to compare the left ventricular repercussion of the PI strategy and PA on the basis of CMR imaging.
CMR is the only technique that currently permits the assessment of the area that was at risk during coronary occlusion, namely the territory displaying edema in T2-weighted sequences.12 Area at risk was identical (29%) in the PI and PA cohorts; this data confirms the similarity between these two groups and permits a head-to-head comparison of their beneficial effects in terms of myocardial salvage.
Late enhancement CMR imaging has emerged as the most reliable method to non-invasively quantify infarct size and microvascular obstruction.11,15 We and others have recently demonstrated that, in STEMI patients, CMR-derived infarct size represents the index most significantly related to prognosis.19,20 With an identical area at risk, PI and PA patients displayed comparable salvaged myocardium and infarct size. Similarly, the extent of microvascular obstruction did not differ between the two strategies.
The equivalent effectiveness of PI and PA regarding infarct size, myocardial savage and microvascular obstruction explains the almost identical results observed with respect to ejection fraction and left ventricular volumes. These variables have been classically considered the main determinants of prognosis in STEMI patients.1,9,10 In turn, these findings could underlie the equivalence of PI and PA in terms of clinical events.
PI and PA patients displayed similar results at the 6-month CMR, emphasizing the beneficial effects of the PI strategy from a long-term perspective.
LimitationsThe main limitation of this study is that it was not randomized. The series of patients treated with each strategy were not concurrent. As a result, the comparison may be confounded by clinical factors, both permanent and time-varying, which could differ between those series.
Patients not treated with reperfusion strategies within the first 12hours after chest pain onset were excluded. Major events or severe clinical instability precluding CMR were also exclusion criteria. Consequently, the final study group comprised a low-to-intermediate risk population and our results can only considered valid in STEMI populations similar to ours.
Consequently, this must be considered as a hypothesis-generating study. A non-inferiority design would be needed to definitively discard significant differences regarding CMR indexes between PI and PA patients. However, this approach would require a huge sample size. For example, on the basis of the results obtained in this study, 11,100 patients should be included to confirm the equivalence of PI and PA in terms of infarct size (alpha=0.05, power=0.09; data obtained with STATA 11, StataCorp, College Station, Texas, USA).
ConclusionsA pharmacoinvasive strategy including thrombolysis, rescue angioplasty if needed and routine but not immediate percutaneous revascularization offers a logistically attractive approach which, in patients without serious complications within the first days after reperfusion, yields the same results as primary angioplasty regarding CMR-derived salvaged myocardium, infarct size, microvascular obstruction, ejection fraction and left ventricular volumes after infarction, in both the short and long term.
FundingThe present study was supported by the “Instituto de Salud Carlos III” (PI080128 and Heracles grants).
Conflicts of InterestNone declared.
Received 24 August 2010
Accepted 15 October 2010
Corresponding author: Departamento de Cardiología, Hospital Clínico Universitario, INCLIVA, Blasco Ibáñez, 17. 46010 Valencia, Spain. vicentbodi@hotmail.com