Severe tricuspid regurgitation (TR) is a highly prevalent clinical entity with high morbidity and mortality rates that predominantly affects women. Given the high surgical risk, treatment has traditionally been medical. However, when left untreated, TR shows rapid progression and a grim prognosis.1–4 Thus, less invasive strategies are being developed, with transcatheter edge-to-edge repair (TEER) being the most frequent percutaneous procedure. However, not all anatomies are suitable for this procedure and, therefore, some orthotopic valves have been studied with good technical success, although the selection criteria remain very strict, and the complication rate is higher than with TEER.4
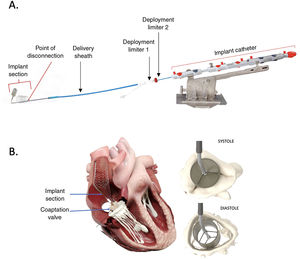
To address these challenges, the CroíValve DUO Tricuspid Coaptation Transcatheter Valve System (CroíValve, Ireland) was developed. The system comprises 2 integrated components (figure 1): a) A 1-size-fits-all coaptation valve (CV) that, unlike previous alternative concepts, incorporates a valve inside to prevent valvular stenosis. The valve has a nitinol frame covered with a porcine pericardium skirt for the native leaflets to coapt against. b) A transjugular system that includes a 22-Fr delivery sheath (through a 26-Fr introducer); a nitinol self-expanding superior vena cava (SVC) stent that anchors the CV in place (available in 3 sizes); an adjustable implant catheter that connects the CV to the SVC stent and remains implanted in the patient; and a delivery system that remains connected to the implant catheter until the end of the procedure, when it is fully disconnected. This design is intended to accommodate any annular diameter and a wide range of right atrial (RA) sizes, while avoiding contact with the atrioventricular node and the right ventricular (RV) free wall.
Early experience with the first device iteration was presented at TVT meeting, which reported 2 device embolizations.5 We present the first case in Spain using the improved device, which includes an increased length of the SVC stent to improve the anchoring. To the best of our knowledge, this is the first publication reporting CroíValve implantation.
The case involved a 79-year-old woman with a prior history of mitral and aortic valve replacement in 2006 due to rheumatic valve disease and massive symptomatic functional TR despite medical treatment. Baseline echocardiography revealed a normal left ventricular ejection fraction, normally functioning mitral and aortic prostheses, enlarged right cavities (basal RV 29mm/m2, RA area 17cm2/m2), preserved RV function (tricuspid annular plane systolic excursion 17mm; fractional area change of 60%), and no evidence of target organ damage or severe pulmonary hypertension (wedge pressure 16mmHg, systolic pulmonary pressure 43mmHg).
After the heart team ruled out a surgical approach, the patient was evaluated for conventional percutaneous therapies such as TEER and other emerging orthotopic prosthesis. However, the anatomy of the valve-right cavities was deemed unsuitable for these treatments. Following discussion with the eligibility committee and after the patient provided informed consent, the decision was made to proceed with the CroíValve system, in accordance with the institution's protocol, which favors investigational orthotopic therapies over bicaval valves as a more physiological solution.
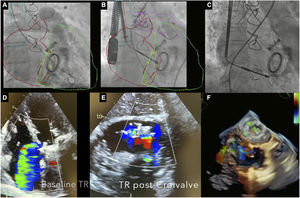
The procedure (video 1 of the supplementary data) was performed under general anesthesia with ultrasound-guided right internal jugular vein puncture and preclosure. A 7-Fr introducer was advanced and a SVC-RA venogram was performed to identify the optimal SVC stent landing zone (figure 2). A 26-Fr introducer was inserted into the RA and an activated clotting time exceeding 300seconds was achieved; the introducer sheath was advanced (under transesophageal echocardiography and fluoroscopy guidance) until the radiopaque marker was visible within the RA. Subsequently, the 22-Fr sheath was introduced until both radiopaque markers were aligned within the RA. Fusion of computed tomography and fluoroscopy imaging assisted in the precise deployment of the CV until the “first deployment limiter” was reached and then the CV was advanced through the tricuspid valve and the catheter angle was adjusted. Then, the self-expanding SVC stent was released by slowly retracting the delivery sheath until reaching the “second deployment limiter”. Up to this point, the stent remained resheathable and repositionable. Then, the position of the CV was readjusted using the controls (knobs) on the delivery catheter handle, modifying the CV height (within the atrium) and its depth (across de tricuspid valve) until optimal TR reduction was achieved. Finally, the system was detached from the implant catheter and the introducer sheath was removed.
A: right ventriculogram and fusion image with computed tomography scan. B: delivery system of the CroíValve with the coaptation valve advanced within the tricuspid annulus. C: final position of the coaptation valve once the superior vena cava was fully deployed. D: baseline degree of tricuspid regurgitation (massive). E: post-CroíValve degree of tricuspid regurgitation (moderate). F. 3-dimensional (3D) echocardiographic image of the final position of the coaptation valve. TR, tricuspid regurgitation.
The patient was discharged 4 days after the procedure with no complications and residual moderate TR (figure 2, video 1 of the supplementary data). At 2 months of follow-up, the patient experienced significant improvement in quality of life (baseline Kansas city cardiomyopathy questionnaire-12 score 53.1 vs 83.3 points) and a slight improvement in functional class (baseline 6-minute walk test 345 meters vs 379.5 meters) with a postexercise Borg dyspnea and fatigue scale of 1 (vs 4 at baseline). The 30-day transthoracic echocardiogram showed a fractional area change of 39% with moderate residual TR medial to the device, between this and the anterior and septal leaflets (video 2 of the supplementary data). Importantly, the patient had no signs of right heart failure and had much better functional status.
With this first experience using the newest iteration device, there is a possibility of achieving improved safety compared with the prior experience reported in the TVT meeting.5 In addition, it is worth noting the simplicity of this device compared with current orthotopic alternatives, as well as the potential short term improvements in imaging parameters and clinical outcomes. Given the high prevalence of severe isolated TR, its high surgical risk and grim prognosis, there is a need for novel percutaneous devices to treat patients not suitable for TEER. This is an isolated case and further data are needed to confirm its safety and efficacy in the long-term. The ongoing TANDEM I study (Identifier: NCT05296148) and future randomized trials will provide further insight.
FUNDINGThe institution received an unconditioned grant as part of the TANDEM-I trial.
ETHICAL CONSIDERATIONSThe protocol was approved by the local ethics committee and the case by the Agencia Española del Medicamento y Dispositivos Sanitarios. Informed consent was obtained both for the intervention and the publication. The sex/gender perspective has been considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools were used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONSI.J. Amat-Santos and S. Blasco-Turrión designed the research and wrote the manuscript. All authors participated in the research and approved the final version of the manuscript.
CONFLICTS OF INTERESTNone.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2023.09.008