The best therapeutic alternative for end-stage heart failure remains heart transplantation (HTx). Nonetheless, because of the severity of the disease and the presence of temporary contraindications, many patients are unsuitable for elective HTx and therefore require short- or long-term circulatory support as a bridge to HTx. Particularly in patients with biventricular heart failure, the circulatory support options before HTx have long been limited to extracorporeal membrane oxygenation (ECMO) and surgical assist devices such as EXCOR (Berlin Heart AG, Germany) and CentriMag (Thoratec Corporation, United States), all of which are associated with a high risk of peritransplant mortality.1,2 Therefore, in recent years, simpler devices have been developed thatvia percutaneous or minimally invasive approaches, can provide temporary circulatory support for the left ventricle or even both ventricles with reduced risk.
The Impella device (Abiomed, United States) is a catheter-based microaxial ventricular assist device. This pump is used in coronary interventions, as well as for cardiogenic shock and as a bridge to HTx.3 Various models are available, such as the Impella 5.0, CP, and 5.5 for left ventricular support and the Impella RP for right ventricular support. The latter is disadvantaged by an exclusively femoral venous access, which impedes patient mobility during extended support periods. This limitation is resolved by the CentriMag device, which can be implanted using a ProtekDuo dual-lumen cannula (LivaNova Plc, United Kingdom) that can be placed via the jugular vein. Here, we describe our initial experience with percutaneous biventricular assist devices (BiVADs) that combine an Impella device and the ProtekDuo cannula in 2 patients with biventricular dysfunction as a bridge to urgent HTx.
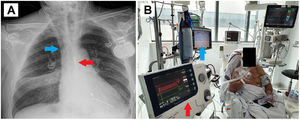
The first case concerns a 60-year-old man on the HTx waiting list due to ischemic dilated cardiomyopathy with severe biventricular dysfunction who experienced decompensation with limiting dyspnea and congestion requiring dobutamine and intense diuretic therapy. The patient exhibited elevated liver and kidney function biomarkers. Despite an initial improvement, he experienced a rebound in liver transaminases and creatinine at 10 days. We decided to implant a BiVAD as a bridge to urgent HTx (figure 1).
A: chest radiograph showing the Impella left ventricular assist device implanted via the right axillary artery (red arrow) and the ProtekDuo cannula and CentriMag right ventricular device implanted via the right jugular vein (blue arrow). B: extubated patient with both support devices performing rehabilitation while on the heart transplant waiting list.
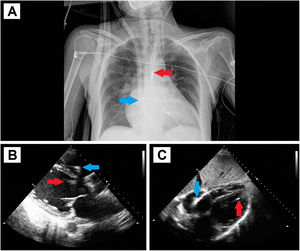
The second case involves a 53-year-old woman with anthracycline-induced dilated cardiomyopathy under treatment with twice-weekly levosimendan who was also on the waiting list for HTx. The patient experienced a sudden deterioration in functional class with hypoperfusion requiring treatment with biogenic amines, diuretic therapy, and ultrafiltration. Given the severe biventricular dysfunction and impossibility of weaning from the applied treatments, we decided to implant a BiVAD (figure 2).
A: chest radiograph showing the Impella left ventricular assist device implanted via the left axillary artery (red arrow) and the ProtekDuo cannula and CentriMag right ventricular device implanted via the right jugular vein (blue arrow). B and C: transthoracic echocardiography with parasternal and subcostal planes for confirming the correct position of the implanted devices.
Both patients showed evidence of multiorgan failure and severe right ventricular dysfunction that prevented implantation of a long-term left ventricular assist device as a bridge to HTx. In both patients, a similar procedure including fluoroscopy guidance and transesophageal echocardiography was followed for BiVAD implantation in a hybrid operating room. First, we used a minimally invasive procedure to implant the Impella 5.0 device for left ventricular support via the axillary artery, applying an 8-mm Dacron graft for anastomosis (right artery in patient 1 and left in patient 2). This required prior study of the artery diameter because it could have been a limiting factor for the implantation. Next, the ProtekDuo cannula was implanted via percutaneous puncture of the right jugular vein and placed in the distal lumen of the pulmonary artery for right ventricular support with a CentriMag device. Both patients were extubated a few hours later and began rehabilitation in a sitting position. No complications occurred. The patients were placed on the waiting list for an urgent HTx once the multiorgan failure had resolved (notably, the creatinine levels of the first patient normalized, reaching 3.3mg/dL). Htx was successfully performed after 21 and 15 days of support, respectively.
In 2019, the first case was reported of percutaneous BiVAD involving the Impella CP and the ProtekDuo cannula in a patient with viral myocarditis.4 This strategy has multiple advantages due to the minimal invasivity of the procedure compared with other devices requiring median sternotomy, which results in fewer transfusions, early extubation, and a more rapid recovery vs other strategies, including minimally invasive implantation techniques involving thoracotomy.5
Another advantage is that a sitting position is possible and even ambulation, which makes this approach the most effective rehabilitation for patients awaiting a HTx. Nonetheless, multiple complications are associated with the devices, particularly due to the vascular access and hemolysis. In our center, before the ProtekDuo cannula became available, double femoral-jugular vein cannulation was performed for right heart support.6 However, the jugular vein access used for the ProtekDuo cannula obviates the need for femoral vein cannulation and facilitates rehabilitation.
In conclusion, BiVAD implantation combining an Impella device implanted through the axillary artery via a minimally invasive approach and the ProtekDuo cannula percutaneously implanted through the jugular vein is a viable option that facilitates the optimal rehabilitation of patients on the HTx waiting list. This approach is associated with a lower incidence of bleeding and infectious complications and less aggressive surgery and could be an alternative in critically ill patients awaiting a HTx.
The present study was approved by the Drug Research Ethics Committee of La Fe University and Polytechnic Hospital. Informed consent was not required because no human experiments were performed and no patient-identifying data are reported. The study complies with current legislation, and this is stated in the favorable report issued by the Drug Research Ethics Committee of La Fe University and Polytechnic Hospital.
FUNDINGWithout any external funding.
AUTHORS’ CONTRIBUTIONSAll authors have contributed to the manuscript conception and design, have drafted the article or critically reviewed its content, have approved the final version for publication, and assume responsibility for all aspects of the article.
CONFLICTS OF INTERESTT. Heredia-Cambra has acted as proctor for the Portico (Abbott), Navitor (Abbott), and MyVal (Meril) platforms and has received honoraria from Palex Medical, Mercé Electromedicina, and Quilpro Cardio. The remaining authors have no conflicts of interest to declare.