Clinical trials have unequivocally shown that cholesterol-lowering drugs decrease the risk of atherosclerotic cardiovascular disease in an exceptionally wide range of individuals. Yet, even when treated optimally according to current standards, many individuals still experience life-threatening ischemic events. Emerging experimental and clinical evidence strongly suggests that persistent inflammation is a major driver of this residual risk, which has opened the door to the application of anti-inflammatory drugs for cardiovascular disease prevention. Here, we review our current knowledge of the biology of interleukin-1β, a key regulator of inflammation in atherosclerotic plaque and the target of the first clinical trial to demonstrate that an anti-inflammatory drug can effectively reduce cardiovascular risk. We discuss the challenges faced by interleukin-1β inhibitors and other anti-inflammatory compounds in their translation to the clinical scenario, and identify other potential targets within this signaling pathway that hold promise in the cardiovascular setting.
Keywords
Since Russell Ross described atherosclerosis as an inflammatory disease in 1999,1,2 thousands of basic research studies have provided accumulating evidence of the clear link between inflammation and atherosclerotic cardiovascular disease (CVD). It is widely accepted that exposure to various cardiovascular risk factors, most notably circulating low density lipoprotein (LDL)-cholesterol, induce a dysfunction of the endothelium that triggers an inflammatory response in the vascular wall. When exposure becomes chronic, this vascular inflammation, unable to neutralize the offending agents, continues indefinitely and, in doing so, leads to the formation, growth and eventual rupture or erosion of the atherosclerotic plaque, the ultimate cause of most ischemic cardiovascular events. Thus, inflammation, while not necessarily a primary trigger of atherogenesis, is an essential intermediary between cardiovascular risk factors and the development of atherosclerosis and its complications. Although our understanding of the intricacies of the inflammatory response in the atherosclerotic plaque remains incomplete, the concept that uncontrolled inflammation is a key driver of atherosclerosis is supported by a myriad of preclinical studies.3–5 Furthermore, biomarkers of systemic inflammation have been associated with an increased risk of cardiovascular events in humans.6,7 Nevertheless, targeting inflammation for CVD prevention was traditionally deemed unfeasible by many researchers and clinicians, given the complex nature of inflammatory responses and the important adverse effects of anti-inflammatory drugs, which hamper their use in chronic disorders. Indeed, nonsteroidal anti-inflammatory drugs, the most commonly used drugs of this kind, increase rather than prevent cardiovascular events.8 This scenario has started to change because of the positive results of the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), which proved for the first time that targeting inflammation can be effective for CVD prevention.9 This focus series of Revista Española de Cardiología aims to provide a brief summary of the current state of the development of anti-inflammatory drugs for CVD prevention. In the present article, we summarize the results of the CANTOS trial and their potential relevance in the clinical setting, and discuss other potential targets related to interleukin 1-beta (IL-1β), the proinflammatory mediator that was inhibited in this clinical trial. An accompanying article describes a number of evolving anti-inflammatory approaches, beyond CANTOS, that are currently being tested in clinical trials for CVD prevention or may become the basis for future trials.
IL-1B: A CENTRAL DRIVER OF INFLAMMATION AND ATHEROGENESISIL-1β, one of the first cytokines to be recognized as such, is a pivotal mediator of cell-to-cell communication within the immune system and a key driver of local and systemic immune responses in atherosclerotic CVD.10 Locally in the vascular wall, IL-1β induces the expression of various cytokines, chemokines and adhesion molecules, which contributes to the recruitment and differentiation of circulating leukocytes.10 Furthermore, IL-1β can also induce its own expression through a positive feedback loop that amplifies the inflammatory response.11–15 Some evidence also suggests that IL-1β promotes a proatherogenic phenotypic shifting in vascular smooth muscle cells by inducing the expression of proinflammatory cytokines, while reducing the expression of smooth muscle markers.16 Systemically, IL-1β contributes to inflammation mainly by inducing the production of the proinflammatory cytokine IL-6, which is known to exert endocrine actions and to elicit an acute-phase response in the liver that can be detected by the presence of C-reactive protein (CRP) in the blood.17 Because of this, CRP, measured by a high sensitivity assay (hsCRP), is widely accepted as a reliable and clinically-relevant biomarker of IL-1β/IL-6-driven systemic inflammation.
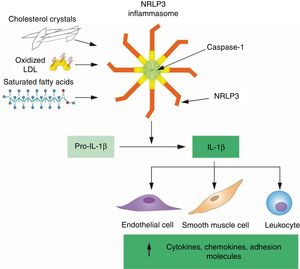
Consistent with its central role in inflammation, IL-1β production and signaling are subject to multiple levels of regulation, which must be considered when designing pharmacological approaches to target this cytokine for CVD prevention. The IL-1β gene is not constitutively expressed and its transcription is only induced upon exposure to proinflammatory stimuli, most typically microbial products or, in the context of the arterial wall, oxidized lipids and lipoproteins that share molecular characteristics with microbial structures and are recognized by the same pattern recognition receptors.18,19 Beyond transcription, IL-1β is also regulated posttranslationally, as it requires proteolytic processing to become biologically active and be secreted to the extracellular space. The main proteolytic enzyme responsible for this cleavage is caspase-1, which undergoes activation by macromolecular signaling platforms known as inflammasomes that only assemble in response to specific stimuli. The triggers of inflammasome activation are manifold and include microbial and nonmicrobial pathogens, crystalline structures, and cell degradation products that are recognized as pathogen-associated or damage-associated molecular patterns by a sensor protein within the inflammasome. Among the various sensor proteins involved in inflammasome activation, NLRP3 is the most relevant in the context of atherosclerotic CVD, as it is activated by various atherogenic lipids, most frequently cholesterol crystals, a by-product of oxidized LDL accumulation in atherosclerotic plaques (Figure 1).20–25 Once secreted, IL-1β function can still be modulated by several competitive inhibitors, such as IL-1 receptor 2 (IL-1R2), a decoy receptor that binds IL-1β, but is unable to signal; or IL-1 receptor antagonist (IL-1RA), a secreted competitive inhibitor of IL-1β binding to its signaling receptor IL-1R1.26
Atherogenic lipids activate the NLRP3 inflammasome to activate IL-1β by proteolytic cleavage. Transcription of the IL1B gene produces pro-IL-1β, a precursor protein that lacks biological activity and is cleaved by caspase 1 to generate the active form of IL-1β, which is secreted to the extracellular space. In the atherosclerotic plaque, caspase 1 activity and IL-1β cleavage depend mainly on the NLRP3 inflammasome, which becomes activated upon sensing atherogenic lipids, most frequently cholesterol crystals. Once secreted, IL-1β induces the expression of various cytokines, chemokines and adhesion molecules central to the perpetuation of inflammation within the atherosclerotic plaque. IL-1β, interleukin 1 beta; LDL, low-density lipoprotein; NLRP3, NLR family pyrin domain containing 3.
A wealth of data from animal studies has demonstrated the overall proatherogenic actions of the NLRP3 inflammasome, IL-1β, and various signaling intermediates related to this cytokine.22,27–35 Although some conflicting results have also been reported,36,37 the direct role of IL-1β in atherogenesis suggested by most of these experimental studies provided a strong basis for the CANTOS clinical trial, which tested whether inhibiting IL-1β-driven inflammation is effective in the secondary prevention of ischemic atherosclerotic events in high-risk patients.
CANTOS: THE ENDGAME OF 2 DECADES OF RESEARCH ON VASCULAR INFLAMMATION IN ATHEROSCLEROSISSince the conception of CANTOS, its results were awaited with great anticipation by the cardiovascular community, as it was meticulously designed as a large-scale proof-of-concept study of the inflammatory hypothesis of atherosclerotic CVD.38
The choice of IL-1β as the target for this first clinical test of the inflammatory basis of atherosclerotic CVD was not random. Many reasons identified IL-1β as an ideal candidate among the myriad cytokines that have been studied in the context of experimental atherosclerosis.39,40 IL-1β and the NLRP3 inflammasome are present at high levels in human atherosclerotic plaques,20,41 and a substantial body of experimental evidence suggests that this cytokine plays a major role in atherogenesis.22,27–35 Importantly, IL-1β inhibition does not affect cholesterol levels,42 in contrast to the inhibition of other proinflammatory cytokines, such as IL-6, which frequently increases LDL cholesterol,43,44 thus challenging its potential as a target for CVD prevention. The existence of several clinically-approved inhibitors of IL-1β, which have shown safety and efficacy in the treatment of rheumatic and autoimmune conditions, also supported the choice of IL-1β as a target. Among them, canakinumab, a human IL-1β-neutralizing monoclonal antibody,45 was chosen for IL-1β inhibition in CANTOS, because of its higher specificity and greater half-life in blood compared with other IL-1β inhibitors, which permitted efficient IL-1β inhibition with subcutaneous doses administered every 3 months. Furthermore, in contrast to other IL-1β inhibitors, canakinumab does not inhibit the related, but functionally distinct cytokine IL-1α. This selectivity is relevant, as recent experimental studies have demonstrated that inhibition of IL-1α or IL-1β can have markedly different effects on atherosclerotic plaque remodeling.34 These characteristics justified the choice of canakinumab over anakinra, a human recombinant form of IL-1RA, which requires daily injections and blocks both IL-1α and IL-1β.
The study population of CANTOS was also carefully selected, as it included exclusively postmyocardial infarction (MI) patients with clear evidence of persistent inflammation, who could be expected to derive significant benefit from treatment with an anti-inflammatory drug. More specifically, CANTOS was a randomized, double-blind, placebo-controlled trial that included ∼10 000 patients with a history of MI and evidence of systemic inflammation (hsCRP ≥ 2mg/L), who were treated according to current standards (including statin therapy) and exhibited low LDL cholesterol levels at baseline (∼80mg/dL). Study participants were randomly allocated to receive placebo or 1 of 3 different doses (50, 150, 300mg) of canakinumab, administered every 3 months.
The main positive finding in CANTOS was a statistically significant 15% relative risk reduction in the primary endpoint–a composite of nonfatal MI, nonfatal stroke, and cardiovascular death–among those allocated to the intermediate 150 mg-dose.9 The 300mg dose had a similar protective effect, which did not reach statistical significance, whereas the 50mg dose of canakinumab was associated with a nonsignificant reduction in relative risk of 7%. Treatment with canakinumab reduced systemic inflammation (hsCRP levels) in a dose-dependent fashion, with no reduction in LDL cholesterol levels, as expected based on previous pilot studies.42 The positive outcomes associated with the higher doses of canakinumab in CANTOS were primarily the result of a reduction in MI and a marked decrease in the need for coronary revascularization procedures (such as angioplasty or bypass surgery), with no significant change in nonfatal stroke or CV death. Additional analyses of CANTOS have recently confirmed the benefits of canakinumab in the prevention of recurrent ischemic events in patients affected by diabetes46 and chronic kidney disease.47 Nevertheless, these benefits come at the price of an important adverse effect: a significant increase in fatal infections. This finding, while expected, highlights the challenges of anti-inflammatory approaches in the setting of chronic conditions, and suggests that careful monitoring will be required for any potential patients treated with canakinumab or similar anti-inflammatory drugs.
Collectively, the positive results of CANTOS represented the endgame of more than 20 years of research, but also the first step in the journey to apply anti-inflammatory therapies to CVD. Accordingly, this trial was received with enthusiasm by researchers and clinicians within the cardiovascular community.48–55 Inescapably, there is now clinical evidence that targeting inflammation–at least IL-1β-driven inflammation–lowers vascular risk in certain high-risk patients independently of changes in circulating lipids. Inflammation can no longer be considered simply as an epiphenomenon, but rather as a driver of atherosclerosis and a potential target for intervention. Therefore, after CANTOS, the doors are open to test a whole host of inflammatory targets. However, whether canakinumab itself will make it to the clinical setting remains unclear.
IL-1B TARGETING WITH CANAKINUMAB: READY FOR THE CLINICAL ARENA?Despite the success of CANTOS, the clinical application of canakinumab faces numerous challenges. While significant, the reduction in absolute risk, even in individuals with persistent systemic inflammation, can be seen as modest: 156 patients would need to be treated with the intermediate dose of canakinumab for 1 year to avoid 1 primary endpoint event.9 This modest benefit needs to be added to the high cost of canakinumab, currently at least 64 000 USD per year considering the CANTOS treatment regime. A recent cost/effectiveness study suggested that a dramatic 98% cost reduction would be needed for canakinumab to be considered cost-effective in secondary CVD prevention.56 Although this compound is now considered an orphan drug and a certain price reduction could be expected if it were approved for CVD prevention, it is unlikely that this will suffice to make it financially viable. Thus, given the fairly modest absolute clinical benefit and its high price, the broad usage of canakinumab in patients with established atherosclerotic CVD is not justified.
The cost-effectiveness of canakinumab could be improved by identifying subgroups of patients who have greater than average reductions in clinical events. In other words, canakinumab could find its clinical niche in the context of personalized medicine. Of note in this regard, a secondary analysis of CANTOS revealed that the magnitude of inflammation inhibition achieved by individual participants was a major determinant of the clinical efficacy of canakinumab. Individuals who achieved hsCRP levels lower than 2mg/L after the initial dose had substantially greater clinical benefits, with relative risk reductions of 25% in major adverse cardiovascular events, in addition to 31% reductions in cardiovascular and all-cause mortality.57 An even bigger benefit was observed in individuals who achieved below the study median levels (1.65 ng/L) of circulating IL-6, a more specific marker of IL-1β-driven inflammation.58 These findings led the CANTOS investigators to propose that on-treatment hsCRP levels after the first dose might be used to identify the patients that would benefit the most from long-term treatment with canakinumab. However, this “responders” approach has some methodological limitations59 and has been reviewed unfavorably by drug regulatory agencies, which have rejected the applications to extend canakinumab indications to secondary CVD prevention based on this analysis.60
Another window of opportunity for the use of canakinumab as a personalized medicine strategy in CVD prevention has opened up with the emergent concept of clonal hematopoiesis and the realization of its pathophysiological relevance in atherosclerotic CVD.61 Sequencing studies have revealed that aging is frequently accompanied by the expansion of hematopoietic clones that carry acquired mutations that provide a competitive advantage to the mutant cell. Between 10% and 20% of healthy individuals aged > 60 years exhibit this somatic mutation-driven clonal hematopoiesis, which results in a substantial fraction of immune cells that carry a mutation with the potential to affect its functionality. Several recent cohort studies indicate that carriers of clonal hematopoiesis-related mutations exhibit a ˃ 2-fold increase in atherosclerotic CVD risk,62,63 and experimental data supports that in some cases this may be linked to exacerbated IL-1β driven inflammation. Two independent studies found that loss of function mutations in TET2, one of the most frequently mutated genes in individuals exhibiting clonal hematopoiesis, accelerate atherosclerosis in parallel with an excess production of IL-1β.63,64 Furthermore, one of these studies showed that suppressing IL-1β production via NLRP3 inflammasome inhibition confers enhanced atheroprotection in mice carrying TET2-mutant cells.64 Thus, mouse studies strongly suggest that IL-1β blockade may be particularly effective for the prevention of CVD in individuals carrying somatic mutations in TET2. Supporting this possibility, an exploratory sequencing analysis of the CANTOS cohort revealed that canakinumab confers a 64% relative risk reduction in carriers of TET2 mutations, which represents a ˃ 4-fold increase in benefit compared with the overall CANTOS population.65 Importantly, this magnitude of CVD risk reduction in a specific population at high risk of atherosclerotic CVD could compensate for the high cost of this drug and justify its use as a personalized preventive care strategy in individuals carrying somatic TET2 mutations. However, this post hoc analysis needs to be interpreted cautiously, and additional clinical trials will be needed to confirm the efficacy of canakinumab in individuals carrying mutations in blood cells in TET2 or other clonal hematopoiesis-related genes.
IL-1B INHIBITION BEYOND ATHEROSCLEROTIC CVDInflammation plays a central role in many age-related conditions and, therefore, anti-inflammatory approaches are of potential value in multiple disorders linked to advanced age. In this regard, emerging evidence, both clinical and experimental, suggest that IL-1β blockade may have applications in nonatherosclerotic conditions both within the cardiovascular field and beyond. Indeed, one of the most fascinating findings in CANTOS was that canakinumab led to a dramatic reduction in the rates of incident lung cancer and cancer mortality, with a remarkable 77% relative risk reduction for fatal lung cancers.66 While exploratory and merely hypothesis-generating, these findings in CANTOS sparked follow-up trials to evaluate whether canakinumab can be an effective adjuvant therapy in the treatment of certain tumors with a known inflammatory basis, such as lung cancer.45,67 Within the cardiovascular setting, results in CANTOS suggest that canakinumab may be effective at limiting acute heart failure episodes,68 a possibility that is also consistent with experimental data.69,70 Interestingly, clonal hematopoiesis has been associated with aggravated heart failure, both in experimental71 and clinical settings,72 further supporting the high potential value of targeting IL-1β in individuals that exhibit somatic mutations linked to this phenomenon. Preclinical evidence also suggests that IL-1β blockade may be of benefit in the context of adverse cardiac remodeling following acute MI.69,73–76 Most recently, IL-1β inhibition shortly after reperfusion has been shown to improve cardiac remodeling and function in a rat ischemia/reperfusion injury model.69 Similarly, a murine surrogate of canakinumab shows benefits when administered after ischemia/reperfusion in an experimental model of acute stroke.77 Collectively, these studies support consideration for additional clinical trials that are adequately powered to evaluate the efficacy of IL-1β inhibition in a variety of cardio/cerebrovascular conditions.
MOVING ON AFTER CANTOS: ALTERNATIVE TARGETS IN THE IL-1B PATHWAYConsidering the success of anti–IL-1β therapy in CANTOS and the challenges that its clinical application faces, it is logical to look for alternative targets in this pathway that might exhibit advantages over canakinumab. One step upstream of IL-1β, the NLRP3 inflammasome deserves special attention as a potential target in atherosclerotic CVD and other inflammatory disorders.78 Inhibiting IL-1β secretion through the highly selective NLRP3 inhibitor MCC95079 has been shown to attenuate atherosclerosis development in hypercholesterolemic mice,80 particularly in conditions of TET2-mutation driven clonal hematopoiesis.64 MCC950 also reduces infarct size and cardiac dysfunction after ischemia/reperfusion in swine models of acute MI,81 and improves cardiac remodeling and function in mouse models of heart failure.71 Importantly, it has been suggested that NLRP3 antagonists may have reduced infection-related adverse-effects compared with direct IL-1β inhibitors, as they may preferentially block dyslipidemia-driven IL-1β secretion, while preserving the activity of other pathogen-recognizing inflammasomes that can be engaged to produce IL-1β in response to infections.82 While this possibility is supported by some experimental data,83 it remains untested clinically. Furthermore, NLRP3 blockade would also impede the release of other signaling molecules cleaved by inflammasomes, such as the IL-18 cytokine. Thus, NLRP3 inhibition may not be equivalent to IL-1β neutralization in terms of immunosuppression, and extensive work is still required to determine whether NLRP3 antagonists are truly advantageous over IL-1β inhibitors for CVD prevention.
Another target of potential value is the cytokine IL-6, a downstream mediator of some of the immunomodulatory actions of IL-1β.17 IL-1β strongly induces IL-6 expression, and the reduction in IL-6 levels is likely an important mechanism of atheroprotection in canakinumab-treated patients.58 However, IL-1β also exerts a number of important IL-6-independent proatherogenic actions, and, therefore, IL-6 inhibition should not be considered equivalent to IL-1β blockade. Indeed, IL-6 inhibitors face unique challenges in the CVD setting because of the complex effects of this cytokine on metabolism. IL-6 exerts conflicting actions on glucose homeostasis and insulin resistance at multiple levels.84–86 Furthermore, as mentioned above, IL-6 inhibition frequently leads to an increase in LDL cholesterol,43,44 which casts doubts on its potential value for atherosclerotic CVD prevention. Nevertheless, efforts are ongoing to develop IL–6-targeted approaches for CVD. A small clinical trial showed that tocilizumab, a monoclonal antibody against the IL-6 receptor, reduces inflammation and troponin release after non–ST-elevation MI,87 suggesting smaller infarct size, an observation that is now being corroborated in the ongoing ASSAIL trial.88
CONCLUSIONSIL-1β inhibition with canakinumab has been the first anti-inflammatory approach to successfully complete the transition from preclinical to clinical studies in atherosclerotic CVD. By doing so, it has validated the inflammatory hypothesis of atherothrombosis and opened the door to the development of novel anti-inflammatory approaches to atherosclerotic CVD, both in the inflammasome/IL-1β signaling pathway and beyond. While unlikely to become broadly used for CVD prevention, IL–1β-targeted therapies may become a powerful tool in precision medicine strategies. It is tempting to envision a future when DNA sequencing data, blood biomarkers, and imaging data are used to identify those individuals who remain at CVD risk even with optimal management of traditional risk factors and who may derive the greatest benefit from anti-inflammatory drugs.
FUNDINGJ.J. Fuster is supported by the Ramón y Cajal program of the Spanish Ministerio de Ciencia, Innovación y Universidades (RYC-2016-20026). The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Ministerio de Ciencia, Innovación y Universidades and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
CONFLICTS OF INTERESTV. Viana-Huete has nothing to disclose. J.J. Fuster is coinventor on a patent related to the treatment of cardiometabolic diseases associated with somatic TET2 mutations.