Valvular heart disease in patients with atrial fibrillation included in clinical trials with direct oral anticoagulants (DOAC) is common and is associated with worse prognosis. The aim of this study was to evaluate the prevalence of valvular heart disease and its influence on clinical events in real-world clinical practice.
MethodsWe conducted a retrospective multicenter registry including 2297 consecutive patients with nonvalvular atrial fibrillation initiating DOAC between January 2013 and December 2016. Valvular heart disease was defined as moderate or severe involvement. The primary study endopoint was the composite of death, stroke or transient ischemic attack/systemic embolism or major bleeding. A competing risks analysis was carried out using a Fine and Gray regression model, with death being the competing event.
ResultsA total of 499 (21.7%) patients had significant valvular heart disease. The most common form was mitral regurgitation (13.7%). Patients with valvular heart disease were older and had more comorbidities. After multivariable analysis, valvular heart disease was associated with a higher risk for the primary endpoint (HR, 1.54; 95%CI, 1.22-1.94; P<.001), death (HR, 1.44; 95%CI, 1.09-1.91, P=.010), and major bleeding (HR, 1.85; 95%CI, 1.23-2.79, P=.003), but there was no association with thromboembolic events (P >.05).
ConclusionsIn patients with nonvalvular atrial fibrillation initiating DOACs, valvular heart disease is common and increases the risk of mortality, stroke, transient ischemic attack/systemic embolism, and major bleeding complications. These findings confirm the results of clinical trials and expand them to a real-life clinical setting.
Keywords
Extensive research has established the efficacy and safety of direct oral anticoagulants (DOAC) in the prevention of stroke and systemic embolism in patients with atrial fibrillation (AF). DOAC therapy is currently recommended over treatment with vitamin K antagonists (VKA), especially for patients initiating anticoagulation.1–7 DOAC therapy has been approved for use in patients with nonvalvular atrial fibrillation (NVAF), a broad category based on the exclusion criteria of several randomized clinical trials (Table 1). However, this terminology has led to therapeutic confusion in clinical practice.8,9 The most recent clinical practice guidelines agree that the current definition of valvular AF relates to AF associated with moderate to severe mitral stenosis (usually rheumatic) or mechanical prosthetic valves.6,7,10
Inclusion Criteria for Clinical Trials of Direct Oral Anticoagulants for Stroke Prevention in Patients With Nonvalvular Atrial Fibrillation
| Classification criteria for nonvalvular atrial fibrillation | |
|---|---|
| RE-LY1 | Excluded: Patients with prosthetic valves, severe mitral stenosis, or valve disease with an indication for intervention before the end of the study |
| Included: Other valve disease: mitral, aortic, or tricuspid regurgitation; aortic stenosis; and mild mitral stenosis | |
| ROCKET-AF2 | Excluded: Patients with hemodynamically significant mitral stenosis, prosthetic valves, or invasive interventions with a high bleeding risk |
| Included: Patients with other valve diseases, annuloplasty (with or without a prosthetic annulus), commissurotomy, and valvuloplasty | |
| ARISTOTLE3 | Excluded: Patients with hemodynamically significant mitral stenosis and prosthetic valves |
| Included: Patients with previous valve surgery in addition to other valve diseases: mitral, aortic, and tricuspid regurgitation; aortic stenosis; and mild mitral stenosis | |
| ENGAGE-AF-TIMI 484 | Excluded: Patients with hemodynamically significant mitral stenosis, mechanical prosthetic valves, or nonresected atrial myxoma |
| Included: Other valve diseases, bioprostheses, or surgical interventions |
Heart valve disease and AF often co-occur and can be independent causes of morbidity and mortality. DOAC clinical trials have included a high proportion of patients with significant valve disease. This subgroup has an unfavorable clinical profile, with outcomes similar to VKA-treated patients.11–15 There is currently little evidence available from real-world experience in patients with heart valve disease and no contraindication for DOAC therapy. The goal of the present study was therefore to analyze the presence of heart valve disease in AF patients initiating DOAC therapy in a real-world setting and to assess its influence on the appearance of events.
METHODSStudy Design and PopulationA retrospective cohort study was conducted to analyze the prevalence of heart valve disease in patients initiating DOAC therapy in real-world clinical practice and to assess the influence of this therapy on the appearance of clinical events. The study included all consecutive NVAF patients receiving a first DOAC prescription at 3 referral centers between January 1, 2013 and December 31, 2016 and who had had an electrocardiogram. Patients prescribed anticoagulation for an indication other than AF were excluded, as were AF patients treated with anticoagulants for electrical or pharmacological cardioversion but who had no indication for long-term oral anticoagulation. Also excluded were patients with hypertrophic cardiomyopathy, moderate to severe rheumatic mitral stenosis, patients with a mechanical prosthetic valve, and patients with a history of DOAC therapy.
Major medical histories were recorded at the start of DOAC therapy. Patients were considered to have significant valve disease if preinclusion echodardiography showed evidence of aortic or mitral valve regurgitation or moderate to severe aortic valve stenosis. All data related to valvular heart disease were collected at the time of inclusion. Disease severity was assessed according to the criteria recommended in European clinical practice guidelines.16,17 All echocardiography studies were reviewed by 2 cardiologists blinded to clinical events, and the opinion of a third cardiologist was sought if there was disagreement. Kidney disease was defined as a glomerular filtration rate <60mL/min/1.73m2 according to the Chronic Kidney Disease Epidemiology Collaboration equation. Anemia was defined as hemoglobin <12g/dL in women and <13g/dL in men. Data were obtained from digitized clinical records held at the participating hospitals and associated primary care centers. The data were recorded by specially trained cardiologists in a bespoke data collection file containing all codified study variables.
This study conformed to the principles of the Declaration of Helsinki.
Follow-up and Outcome VariablesPatients were followed up from the date of DOAC prescription to the end of the study period, a median of 606 days [interquartile range, 474-731 days]. Follow-up was completed for 99.7% of patients (n=2290). The principal outcome measure was the composite of death, stroke/transient ischemic attack (TIA)/systemic embolism, and major bleeding. Component events of the main outcome measure were also recorded separately during follow-up. Cause of death was categorized as cardiovascular, noncardiovascular, or undetermined according to previously defined criteria.18 Stroke was defined as signs or symptoms of neurological dysfunction secondary to a central nervous system infarction. TIA was defined as signs or symptoms of neurological dysfunction lasting for less than 24hours in the absence of a lesion detectable with neuroimaging techniques.18 Major bleeding was defined according to the International Society on Thrombosis and Haemostasis criteria: fatal bleeding, symptomatic bleeding in a critical area or organ (such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome), or bleeding causing a drop in hemoglobin of ≥20g/L or requiring transfusion of 2 or more units of whole blood or red cells.19
Statistical AnalysisFor the analysis of patient characteristics, quantitative variables are expressed as mean ±standard deviation or median [interquartile range] and categorical variables as absolute or relative frequencies. Continuous variables were compared by the Student t test or ANOVA, and categorical variables were compared by the Pearson chi-square test.
To identify factors associated with the studied events, hazard ratios (HR) were calculated by Cox multivariate regression analysis. Because death is a competing risk with embolism and bleeding events, we used the Fine and Gray competing risk model to estimate event incidence and identify predictive factors.20 The independent variables included in the Cox regression models were those showing an association with clinical events in the univariate analysis and judged by the investigators as important for the adjustment. Data collection was preceded by a comprehensive literature search to identify the major variables associated with each event. In addition, the linearity assumption was assessed using Martingale residuals. The proportional risk assumption was verified graphically and statistically for all Cox regression analyses, independently of event type (primary event, death, or competing events). A detailed breakdown of the variables included in the Cox univariate regression analysis is provided in . Differences were considered statistically significant at P <.05. The statistical analysis was performed with the statistical packages SPSS v21 (SPSS Inc; Chicago, Illinois, United States) and STATA v13.0 (Stata Corp LP.; Texas, United States).
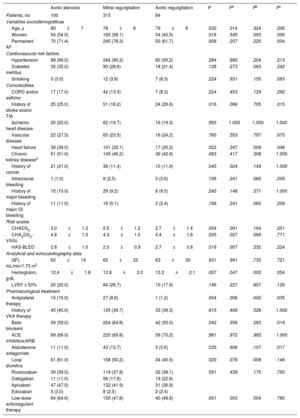
RESULTSIn total, 2297 patients were included, of whom 915 (39.8%) received rivaroxaban, 419 (18.2%) dabigatran, 896 (39.0%) apixaban, and 67 (2.9%) edoxaban. Of the total study population, 499 patients (21.7%) had significant heart valve disease. Patients with valve disease tended to be older and have more comorbidity, and thus scored higher on thromboembolic and bleeding risk scales (CHA2DS2-VASc, 4.4 ±1.5 vs 4.0 ±1.7; P <.001; HAS-BLED, 2.6±0.9 vs 2.4±1.0; P <.001). The presence of heart valve disease showed no association with the type of DOAC prescribed (P>.05) (Table 2).
Patient Clinical Characteristics According to the Presence of Heart Valve Disease
| Total | No heart valve disease | Significant heart valve disease | P | |
|---|---|---|---|---|
| Patients, no. | 2297 | 1798 | 499 | |
| Sociodemographic variables | ||||
| Age, y | 76±10 | 75±10 | 79±8 | <.001 |
| Women | 1216 (52.9) | 945 (52.6) | 271 (54.3) | .521 |
| Permanent AF | 1309 (57.7) | 944 (53.2) | 365 (74.2) | <.001 |
| Cardiovascular risk factors | ||||
| Hypertension | 1999 (87.0) | 1546 (86.0) | 453 (90.8) | .006 |
| Diabetes mellitus | 741 (32.3) | 598 (33.3) | 143 (28.7) | .058 |
| Smoking | 146 (6.4) | 124 (6.9) | 22 (4.4) | .048 |
| Comorbidities | ||||
| COPD and/or asthma | 321 (14.0) | 255 (14.2) | 66 (13.2) | .637 |
| History of stroke and/or TIA | 470 (20.5) | 370 (20.6) | 100 (20.0) | .841 |
| Ischemic heart disease | 384 (16.7) | 286 (15.9) | 98 (19.7) | .054 |
| Vascular disease | 424 (22.0) | 321 (21.3) | 103 (24.3) | .201 |
| Heart failure | 449 (19.5) | 292 (16.2) | 157 (31.5) | <.001 |
| Chronic kidney diseasea | 818 (36.1) | 586 (33.2) | 232 (46.7) | <.001 |
| History of cancer | 278 (12.1) | 211 (11.7) | 67 (13.4) | .346 |
| Intracranial bleeding | 60 (2.6) | 48 (2.7) | 12 (2.4) | .190 |
| History of major bleeding | 200 (8.7) | 148 (8.2) | 52 (10.4) | .149 |
| History of major GI bleeding | 89 (3.9) | 60 (3.3) | 29 (5.8) | .190 |
| Previous labile INRb | 425 (68.8) | 348 (68.6) | 77 (69.4) | .970 |
| Risk scales | ||||
| CHADS2 | 2.4±1.3 | 2.4±1.3 | 2.7±1.2 | <.001 |
| CHA2DS2-VASc | 4.1±1.6 | 4.0±1.7 | 4.4±1.5 | <.001 |
| HAS-BLED | 2.4±1.0 | 2.4±1.0 | 2.6±0.9 | <.001 |
| Analytical and echocardiography data | ||||
| GFR, mL/min/1.73m2 | 68±20 | 70±20 | 63±21 | <.001 |
| Hemoglobin, g/dL | 13.4±1.9 | 13.5±1.9 | 12.8±2.0 | <.001 |
| LVEF ≤ 50% | 310 (13.8) | 191 (10.9) | 119 (23.8) | <.001 |
| Pharmacological treatment | ||||
| Aspirin | 183 (8.0) | 148 (8.2) | 35 (7.0) | .423 |
| Antiplatelet therapy | 228 (9.9) | 185 (10.3) | 43 (8.6) | .307 |
| History of VKA therapy | 1019 (44.4) | 816 (45.5) | 203 (40.7) | .064 |
| Beta-blockers | 1332 (58.0) | 1027 (57.2) | 305 (61.1) | .127 |
| ACE inhibitors/ARB | 1545 (67.3) | 1197 (66.6) | 348 (69.7) | .212 |
| Aldosterone antagonists | 157 (6.8) | 100 (5.6) | 57 (11.4) | <.001 |
| Loop diuretics | 824 (35.9) | 571 (31.8) | 253 (50.7) | <.001 |
| Rivaroxaban | 915 (39.8) | 725 (40.3) | 190 (38.1) | .456 |
| Dabigatran | 419 (18.2) | 333 (18.5) | 86 (17.2) | |
| Apixaban | 896 (39.0) | 686 (38.2) | 210 (42.1) | |
| Edoxaban | 67 (2.9) | 54 (3.0) | 13 (2.6) | |
| Low-dose anticoagulant therapy | 915 (40.0) | 661 (36.9) | 254 (51.3) | <.001 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; GI, gastrointestinal; INR, international normalized ratio; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; VKA, vitamin K antagonists.
Data are expressed as mean±standard deviation or No. (%).
Clinical characteristics were analyzed according to the predominant valve disease (Table 3). The most frequent valve disease was mitral regurgitation (315 patients; 13.7%), followed by aortic stenosis (100 patients; 4.4%) and aortic regurgitation (84 patients; 3.7%). Population characteristics are stratified according to valve disease type in Table 3.
Patient Characteristics According to Valve Disease Type
| Aortic stenosis | Mitral regurgitation | Aortic regurgitation | P | Pa | Pb | Pc | |
|---|---|---|---|---|---|---|---|
| Patients, no. | 100 | 315 | 84 | ||||
| Variables sociodemográficas | |||||||
| Age, y | 80±7 | 78±9 | 79±8 | .032 | .014 | .324 | .206 |
| Women | 54 (54.0) | 183 (58.1) | 34 (40.5) | .016 | .545 | .093 | .006 |
| Permanent AF | 70 (71.4) | 245 (78.3) | 50 (61.7) | .008 | .207 | .225 | .004 |
| Cardiovascular risk factors | |||||||
| Hypertension | 89 (89.0) | 284 (90.2) | 80 (95.2) | .284 | .885 | .204 | .213 |
| Diabetes mellitus | 35 (35.0) | 90 (28.6) | 18 (21.4) | .128 | .273 | .063 | .242 |
| Smoking | 3 (3.0) | 12 (3.8) | 7 (8.3) | .224 | .931 | .155 | .093 |
| Comorbidities | |||||||
| COPD and/or asthma | 17 (17.0) | 42 (13.3) | 7 (8.3) | .224 | .453 | .129 | .292 |
| History of stroke and/or TIA | 25 (25.0) | 51 (16.2) | 24 (28.6) | .016 | .066 | .705 | .015 |
| Ischemic heart disease | 20 (20.0) | 62 (19.7) | 16 (19.3) | .993 | 1.000 | 1.000 | 1.000 |
| Vascular disease | 22 (27.5) | 65 (23.5) | 16 (24.2) | .760 | .553 | .797 | .675 |
| Heart failure | 39 (39.0) | 101 (32.1) | 17 (20.2) | .022 | .247 | .009 | .048 |
| Chronic kidney diseased | 51 (51.0) | 145 (46.2) | 36 (42.9) | .483 | .417 | .308 | 1.000 |
| History of cancer | 21 (21.0) | 36 (11.4) | 10 (11.9) | .045 | .024 | .149 | 1.000 |
| Intracranial bleeding | 1 (1.0) | 8 (2.5) | 3 (3.6) | .156 | .241 | .060 | .209 |
| History of major bleeding | 15 (15.0) | 29 (9.2) | 8 (9.5) | .245 | .146 | .371 | 1.000 |
| History of major GI bleeding | 11 (11.0) | 16 (5.1) | 2 (2.4) | .156 | .241 | .060 | .209 |
| Risk scales | |||||||
| CHADS2 | 3.0±1.2 | 2.5±1.2 | 2.7±1.4 | .004 | .001 | .164 | .201 |
| CHA2DS2-VASc | 4.8±1.5 | 4.3±1.5 | 4.4±1.6 | .025 | .007 | .069 | .771 |
| HAS-BLED | 2.8±1.0 | 2.5±0.9 | 2.7±0.8 | .018 | .007 | .232 | .224 |
| Analytical and echocardiography data | |||||||
| GFI, mL/min/1.73 m2 | 62±19 | 62±22 | 63±20 | .931 | .991 | .735 | .721 |
| Hemoglobin, g/dL | 12.4±1.8 | 12.8±2.0 | 13.3±2.1 | .007 | .047 | .002 | .054 |
| LVEF ≤ 50% | 20 (20.0) | 84 (26.7) | 15 (17.9) | .146 | .227 | .857 | .129 |
| Pharmacological treatment | |||||||
| Antiplatelet therapy | 15 (15.0) | 27 (8.6) | 1 (1.2) | .004 | .096 | .002 | .035 |
| History of VKA therapy | 45 (45.0) | 125 (39.7) | 33 (39.3) | .615 | .409 | .528 | 1.000 |
| Beta-blockers | 59 (59.0) | 204 (64.8) | 42 (50.0) | .042 | .356 | .283 | .019 |
| ACE inhibitors/ARB | 69 (69.0) | 220 (69.8) | 59 (70.2) | .981 | .972 | .983 | 1.000 |
| Aldosterone antagonists | 11 (11.0) | 43 (13.7) | 3 (3.6) | .035 | .606 | .107 | .017 |
| Loop diuretics | 61 (61.0) | 158 (50.2) | 34 (40.5) | .020 | .076 | .009 | .146 |
| Rivaroxaban | 39 (39.0) | 119 (37.8) | 32 (38.1) | .551 | .439 | .175 | .750 |
| Dabigatran | 11 (11.0) | 56 (17.8) | 19 (22.6) | ||||
| Apixaban | 47 (47.0) | 132 (41.9) | 31 (36.9) | ||||
| Edoxaban | 3 (3.0) | 8 (2.5) | 2 (2.4) | ||||
| Low-dose anticoagulant therapy | 64 (64.6) | 150 (47.8) | 40 (48.8) | .001 | .003 | .004 | .785 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; GI, gastrointestinal; INR, international normalized ratio; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; VKA, vitamin K antagonists.
Data are expressed as mean±standard deviation or No. (%).
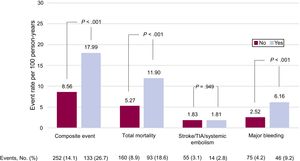
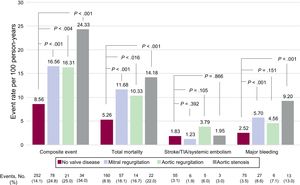
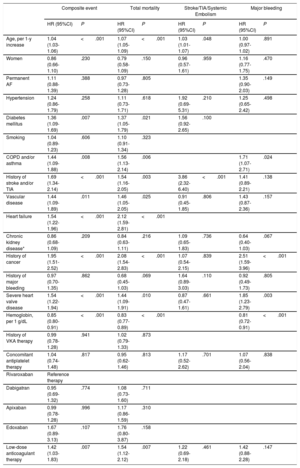
Clinical events recorded during follow-up (606 [474-731] days) are classified according to the presence and type of valve disease in Figure 1 and Figure 2. The presence of valve disease was associated with a higher total event rate: composite event, 17.99 vs 8.56 per 100 person-years (P>.001); death, 11.90 vs 5.27 per 100 person-years (P>.001); and major bleeding, 6.16 vs 2.52 per 100 person-years (P>.001). However, valve disease showed no association with thromboembolic events (ischemic stroke/TIA/ systemic embolism: 1.81 vs 1.83 per100 person-years; P>.05). Similarly, all types of heart valve disease analyzed were associated with higher rates for the composite event, death, and major bleeding, although for aortic regurgitation the association did not reach statistical significance. None of the embolic events analyzed showed an association with higher risk (Figure 2).
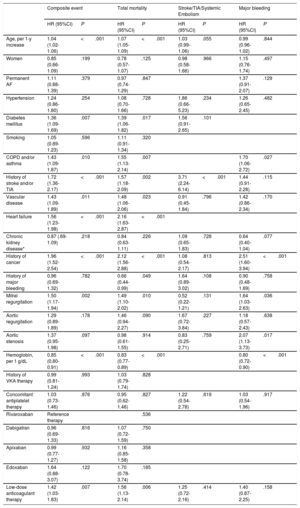
The univariate Cox proportional risk analysis for the prediction of each event is summarized in . Multivariate adjustment revealed significant valvular heart disease as an independent predictor of the composite event (HR=1.54; 95% confidence interval [95%CI], 1.22-1.94; P <.001), death (HR=1.44; 95%CI, 1.09-1.91; P=.010), and major bleeding (HR=1.85; 95%CI, 1.23-2.79; P=.003). However, the presence of valve disease was not a predictor of stroke/TIA and/or systemic embolism (P>.05) (Table 4A). The composite event, death, and major bleeding were also independently predicted by mitral valve regurgitation and aortic valve stenosis (Table 4B). Finally, discrimination analysis of the regression models showed c-statistics between 0.71 and 0.80 (detail in Table 4A-B).
Multivariate Cox Proportional Risk Analysis for the Prediction of the Composite Event, Total Mortality, Stroke/TIA/Systemic Embolism, and Bleeding
| Composite event | Total mortality | Stroke/TIA/Systemic Embolism | Major bleeding | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Age, per 1-y increase | 1.04 (1.03-1.06) | <.001 | 1.07 (1.05-1.09) | <.001 | 1.03 (1.01-1.07) | .048 | 1.00 (0.97-1.02) | .891 |
| Women | 0.86 (0.66-1.10) | .230 | 0.79 (0.58-1.09) | .150 | 0.96 (0.57-1.61) | .959 | 1.16 (0.77-1.75) | .470 |
| Permanent AF | 1.11 (0.88-1.39) | .388 | 0.97 (0.73-1.28) | .805 | 1.35 (0.90-2.03) | .149 | ||
| Hypertension | 1.24 (0.86-1.79) | .258 | 1.11 (0.73-1.71) | .618 | 1.92 (0.69-5.31) | .210 | 1.25 (0.65-2.42) | .498 |
| Diabetes mellitus | 1.36 (1.09-1.69) | .007 | 1.37 (1.05-1.79) | .021 | 1.56 (0.92-2.65) | .100 | ||
| Smoking | 1.04 (0.89-1.23) | .606 | 1.10 (0.91-1.34) | .323 | ||||
| COPD and/or asthma | 1.44 (1.09-1.88) | .008 | 1.56 (1.13-2.14) | .006 | 1.71 (1.07-2.71) | .024 | ||
| History of stroke and/or TIA | 1.69 (1.34-2.14) | <.001 | 1.54 (1.16-2.05) | .003 | 3.86 (2.32-6.40) | <.001 | 1.41 (0.89-2.21) | .138 |
| Vascular disease | 1.44 (1.09-1.89) | .011 | 1.46 (1.05-2.05) | .025 | 0.91 (0.45-1.85) | .806 | 1.43 (0.87-2.36) | .157 |
| Heart failure | 1.54 (1.22-1.96) | <.001 | 2.12 (1.59-2.81) | <.001 | ||||
| Chronic kidney disease* | 0.86 (0.68-1.09) | .209 | 0.84 (0.63-1.11) | .216 | 1.09 (0.65-1.83) | .736 | 0.64 (0.40-1.03) | .067 |
| History of cancer | 1.95 (1.51-2.52) | <.001 | 2.08 (1.54-2.83) | <.001 | 1.07 (0.54-2.15) | .839 | 2.51 (1.59-3.96) | <.001 |
| History of major bleeding | 0.97 (0.70-1.35) | .862 | 0.68 (0.45-1.03) | .069 | 1.64 (0.89-3.03) | .110 | 0.92 (0.49-1.73) | .805 |
| Severe heart valve disease | 1.54 (1.22-1.94) | <.001 | 1.44 (1.09-1.91) | .010 | 0.87 (0.47-1.61) | .661 | 1.85 (1.23-2.79) | .003 |
| Hemoglobin, per 1 g/dL | 0.85 (0.80-0.91) | <.001 | 0.83 (0.77-0.89) | <.001 | 0.81 (0.72-0.91) | <.001 | ||
| History of VKA therapy | 0.99 (0.78-1.28) | .941 | 1.02 (0.79-1.33) | .873 | ||||
| Concomitant antiplatelet therapy | 1.04 (0.74-1.48) | .817 | 0.95 (0.62-1.46) | .813 | 1.17 (0.52-2.62) | .701 | 1.07 (0.56-2.04) | .838 |
| Rivaroxaban | Reference therapy | |||||||
| Dabigatran | 0.95 (0.69-1.32) | .774 | 1.08 (0.73-1.60) | .711 | ||||
| Apixaban | 0.99 (0.78-1.28) | .996 | 1.17 (0.86-1.59) | .310 | ||||
| Edoxaban | 1.67 (0.89-3.13) | .107 | 1.76 (0.80-3.87) | .158 | ||||
| Low-dose anticoagulant therapy | 1.42 (1.03-1.83) | .007 | 1.54 (1.12-2.12) | .007 | 1.22 (0.69-2.18) | .461 | 1.42 (0.88-2.28) | .147 |
Multivariate Cox Proportional Risk Analysis for the Prediction of the Composite Event, Total Mortality, Stroke/TIA/Systemic Embolism, and Bleeding
| Composite event | Total mortality | Stroke/TIA/Systemic Embolism | Major bleeding | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Age, per 1-y increase | 1.04 (1.02-1.06) | <.001 | 1.07 (1.05-1.09) | <.001 | 1.03 (0.99-1.06) | .055 | 0.99 (0.96-1.02) | .844 |
| Women | 0.85 (0.66-1.09) | .199 | 0.78 (0.57-1.07) | .125 | 0.98 (0.58-1.66) | .966 | 1.15 (0.76-1.74) | .497 |
| Permanent AF | 1.11 (0.88-1.39) | .379 | 0.97 (0.74-1.29) | .847 | 1.37 (0.91-2.07) | .129 | ||
| Hypertension | 1.24 (0.86-1.80) | .254 | 1.08 (0.70-1.66) | .728 | 1.86 (0.66-5.23) | .234 | 1.26 (0.65-2.45) | .482 |
| Diabetes mellitus | 1.36 (1.09-1.69) | .007 | 1.39 (1.06-1.82) | .017 | 1.56 (0.91-2.65) | .101 | ||
| Smoking | 1.05 (0.89-1.23) | .596 | 1.11 (0.91-1.34) | .320 | ||||
| COPD and/or asthma | 1.43 (1.09-1.87) | .010 | 1.55 (1.13-2.14) | .007 | 1.70 (1.06-2.72) | .027 | ||
| History of stroke and/or TIA | 1.72 (1.36-2.17) | <.001 | 1.57 (1.18-2.09) | .002 | 3.71 (2.24-6.14) | <.001 | 1.44 (0.91-2.28) | .115 |
| Vascular disease | 1.43 (1.09-1.89) | .011 | 1.48 (1.06-2.06) | .023 | 0.91 (0.45-1.84) | .796 | 1.42 (0.86-2.34) | .170 |
| Heart failure | 1.56 (1.23-1.98) | <.001 | 2.16 (1.63-2.87) | <.001 | ||||
| Chronic kidney disease* | 0.87 (.69-1.09) | .218 | 0.84 (0.63-1.11) | .226 | 1.09 (0.65-1.83) | .728 | 0.64 (0.40-1.04) | .077 |
| History of cancer | 1.96 (1.52-2.54) | <.001 | 2.12 (1.56-2.88) | <.001 | 1.08 (0.54-2.17) | .813 | 2.51 (1.60-3.94) | <.001 |
| History of major bleeding | 0.96 (0.69-1.32) | .782 | 0.66 (0.44-0.99) | .049 | 1.64 (0.89-3.02) | .108 | 0.90 (0.48-1.69) | .758 |
| Mitral regurgitation | 1.50 (1.17-1.94) | .002 | 1.49 (1.10-2.02) | .010 | 0.52 (0.22-1.21) | .131 | 1.64 (1.03-2.63) | .036 |
| Aortic regurgitation | 1.29 (0.89-1.89) | .178 | 1.46 (0.94-2.27) | .090 | 1.67 (0.72-3.84) | .227 | 1.18 (0.57-2.43) | .638 |
| Aortic stenosis | 1.37 (0.95-1.98) | .097 | 0.98 (0.61-1.55) | .914 | 0.83 (0.25-2.71) | .759 | 2.07 (1.13-3.73) | .017 |
| Hemoglobin, per 1 g/dL | 0.85 (0.80-0.91) | <.001 | 0.83 (0.77-0.89) | <.001 | 0.80 (0.72-0.90) | <.001 | ||
| History of VKA therapy | 0.99 (0.81-1.24) | .993 | 1.03 (0.79-1.74) | .828 | ||||
| Concomitant antiplatelet therapy | 1.03 (0.73-1.46) | .876 | 0.95 (0.62-1.46) | .827 | 1.22 (0.54-2.78) | .619 | 1.03 (0.54-1.96) | .917 |
| Rivaroxaban | Reference therapy | .536 | ||||||
| Dabigatran | 0.96 (0.69-1.33) | .816 | 1.07 (0.72-1.59) | .750 | ||||
| Apixaban | 0.99 (0.77-1.27) | .932 | 1.16 (0.85-1.58) | .358 | ||||
| Edoxaban | 1.64 (0.88-3.07) | .122 | 1.70 (0.78-3.74) | .185 | ||||
| Low-dose anticoagulant therapy | 1.42 (1.03-1.83) | .007 | 1.56 (1.13-2.14) | .006 | 1.25 (0.72-2.16) | .414 | 1.40 (0.87-2.25) | .158 |
95%CI, 95% confidence interval; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; TIA, transient ischemic attack; VKA, vitamin K antagonists.
Multivariate model for the composite of death, stroke/TIA/systemic embolism, and major bleeding; c-statistic=0.77 (0.74-0.79).
Multivariate model for total mortality; c-statistic=0.80 (0.78-0.83).
Multivariate model for stroke/TIA/systemic embolism; c-statistic=0.72 (0.67-0.78).
Multivariate models for major bleeding; c-statistic=0.71 (0.67-0.76).
The present study evaluated the frequency of heart valve disease and its prognostic value in a contemporary cohort of NVAF patients initiating DOAC therapy. This patient population had a high prevalence of significant valve disease, and this was associated with an elevated risk of adverse events during follow-up. This Spanish multicenter registry is the first to evaluate the frequency and prognostic value of heart valve disease in NVAF patients in a real-world setting, outside the context of a randomized clinical trial. We therefore considered that it would provide important clinical information.
In recent years, the definition of “valvular” AF has provoked controversy, probably due in part to the widely heterogeneous exclusion criteria used in different DOAC clinical trials in AF patients (Table 1).21–24 The appropriateness of the term valvular AF remains a subject of debate. Some authors propose the alternative term ‘mechanical and rheumatic mitral atrial fibrillation’ to describe the condition of patients for whom DOAC therapy is not indicated. Similarly, European guidelines propose the replacement of the current terminology with a classification based on the underlying specific valve disease. In the proposed definition, valve diseases are classified into 2 groups based on the indicated anticoagulant therapy: type 1 disease corresponds to AF patients with heart valve disease requiring VKA therapy (moderate to severe rheumatic mitral stenosis or mechanical valve prosthesis); type 2 disease corresponds to AF patients with heart valve disease requiring therapy with a VKA or a DOAC, also taking into consideration the thromboembolic risk.9,10 Given that valve disease affects up to 30% of AF patients, it would seem prudent, in the absence of consensus, to use the definition provided in the clinical practice guidelines.6,7,10,25
In the present study, the presence of heart valve disease in AF patients was associated with a worse clinical profile, in line with clinical trial substudies.11–14 Similarly, AF patients with significant valve disease treated in real-world clinical practice tended to be older and were more likely than unaffected patients to have ischemic heart disease, a history of major bleeding, heart failure, and renal deterioration. Although this profile would seem to predict higher scores on the main risk scales, published clinical trial substudies present an inconsistent picture. In the ARISTOTLE and ENGAGE-AF trials, patients with heart valve disease had significantly higher scores on the CHADS2 scale than those who did not (2.2 vs 2.1 and 2.9 vs 2.8, respectively; P <.001); however, no such differences were found in the RE-LY and ROCKET-AF studies.11–14 Scores on the HAS-BLED scale in AF patients were recorded in only 2 trials, and the results were once again inconsistent. Whereas the ENGAGE-AF trial found an increased bleeding risk among AF patients with valve disease (2.6 vs 2.5; P=.018), the ROCKET-AF trial showed no effect (2.8 vs 2.8; P=.18).12,14 In the present study, patients with heart valve disease had a higher thrombotic risk and a statistically nonsignificant tendency toward a higher bleeding risk (CHA2DS2-VASc, 3.9±1.6 vs 4.4±1.6; P <.001; HAS-BLED, 1.6±0.9 vs 1.8±0.9; P=.060).
In the present series, the presence of valve disease was one of the most notable independent predictors of death and bleeding complications. In contrast, valve disease showed no association with the risk of thromboembolic events, even though these patients had high scores on thrombotic risk scales. These findings are in broad agreement with the results of clinical trial substudies, especially the ENGAGE-AF trial.14 Patients with heart valve disease in the RE-LY and ROCKET-AF trials had a similar risk of stroke/systemic embolism and death, with an increase in the bleeding risk.11,12 Moreover, the risk of stroke/systemic embolism and death was higher among patients with heart valve disease in the ARISTOTLE trial, whereas there was no evidence of a differential effect on bleeding risk.13 A subsequent meta-analysis including all 4 substudies produced results consistent with the present study.15
The close relationship between valvular heart disease and adverse events during follow-up suggests that valve functional status should be a factor in risk stratification and the design of strategies to improve prognosis in these patients. The mechanisms linking significant valve disease to clinical events remain unclear. AF worsens the prognosis of patients with severe heart valve disease; moreover, valve disease and AF mutually reinforce each other through volume-pressure overload and neurohormonal factors.26–28 It is for this reason, when valve dysfunction is severe, that AF can be considered a marker of progressive disease. Heart valve disease is a major independent predictor of mortality in AF patients, as observed in the present study; in light of this strong association, valve repair or replacement is recommended to reduce mortality in this patient subgroup.29 Moreover, aortic stenosis is known to be associated with gastrointestinal bleeding, which has a major impact on quality of life, hospital admissions, and mortality. Bleeding and angiodysplasia in Heyde syndrome are a consequence of acquired Von Willebrand factor deficiency secondary to the shear forces generated on the surface of the stenotic and calcified aortic valve; even moderate shear forces trigger von Willebrand factor proteolysis.30 Given the bleeding risk associated with oral anticoagulant therapy, it is of fundamental importance to stratify bleeding risk in patients initiating this therapy and to modify factors that increase bleeding risk in this population. Established factors that increase this bleeding risk include concomitant antiplatelet therapy, nonsteroidal antiinflammatory drugs, and poorly controlled hypertension.31
LimitationsThe main limitations of this study are related to its retrospective design. However, strengths of the study include its multicenter nature, the number of recorded events, and the low number of patients lost to follow-up. Moreover, these registry data were collected by specifically trained cardiologists and were not obtained from administrative or insurance company databases, strengthening the reliability of the results obtained. In addition, to date there has been little available information on the use of DOAC therapy in NVAF patients in Spain, further supporting the importance of these findings. The major clinical trial substudies showed a similar overall DOAC efficacy and safety in AF patients with or without heart valve disease; however, this question could not be addressed in the present study due to the lack of a control group of VKA-treated patients.15
CONCLUSIONSNVAF patients initiating DOAC therapy frequently have co-occurring heart valve disease, and this is associated with an elevated risk of death, stroke/TIA/systemic embolism, and bleeding complications. These findings not only confirm the results of clinical trials in similar patient populations, but also extend them to real-world clinical practice.
CONFLICTS OF INTERESTNone declared.
- –
Heart valve disease is highly prevalent in AF patients included in clinical trials of DOAC therapy.
- –
Several substudies of these clinical trials have shown an association between heart valve disease and poor prognosis, although valve function does not affect the advantages of DOAC therapy in this context.
- –
There is little available evidence on the prevalence or prognostic impact of heart valve disease in patients with NVAF initiating DOAC therapy outside of the clinical trial setting.
- –
Heart valve disease is common in NVAF patients initiating DOAC therapy in real-world clinical practice.
- –
Heart valve disease is associated with an unfavorable clinical profile and an increased risk of death and severe bleeding complications but shows no association with thromboembolic events.
- –
The current findings confirm clinical trial results in a real-world clinical context.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2018.08.026.